Reproducible synthesis of high quality MCM-48 by extraction and recuperation of the gemini surfactant
M. Benjelloun, P. Van Der Voort*, P. Cool, O. Collart and E. F. Vansant
Uni![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ersity of Antwerpen (U.I.A.), Department of Chemistry, Laboratory of Adsorption and Catalysis, Uni
ersity of Antwerpen (U.I.A.), Department of Chemistry, Laboratory of Adsorption and Catalysis, Uni![[italic v]](https://www.rsc.org/images/entities/char_e0f5.gif) ersiteitsplein 1, 2610, Wilrijk, Belgium. E-mail: pascal.vandervoort@ua.ac.be
ersiteitsplein 1, 2610, Wilrijk, Belgium. E-mail: pascal.vandervoort@ua.ac.be
First published on 13th December 2000
Abstract
High quality MCM-48 can be repeatedly and reproducibly prepared, as a novel method for the extraction, recuperation and re-use of the gemini surfactant has been optimized. Gemini surfactants have the general formula [CnH2n+1N+(CH3)2(CH2)sN+(CH3)2CmH2m+1]·2Br, and have been shown to produce MCM-48 in a very convenient way. In each cycle, more than 95 wt% of the surfactant is extracted under mild conditions. The extracted surfactant does not degenerate during this treatment and can be re-used many times. The MCM-48 produced by this soft extraction, has a narrower mesoporous pore size distribution and a larger pore radius than materials prepared by normal temperature-programmed calcination. The surface of the extracted MCM-48 still contains sufficient silanol groups, although some silanol groups have been replaced by methoxy groups. The number of silanols on the surface of the MCM-48 has been titrated by surface reaction with hexamethyldisilazane, followed by quantification of the liberated NH3.
Introduction
MCM-48 (the cubic Ia3d member of the M41S family) can be considered as one of the more promising materials for adsorption and catalysis. In general, it has a surface area exceeding 1500 m2 g−1, pore volumes >1 ml g−1 and a very narrow, but tuneable mesoporous pore size distribution. Moreover, it has a unique three dimensional structure, which makes the pore system available in three dimensions. For these reasons, MCM-48 is a candidate for membrane reactors or as a catalytic support.One of the major drawbacks for the industrial use of these materials is their high synthesis cost and the poor reproducibility of the synthesis procedure. In the last few years, the introduction of the gemini surfactants, followed by a hydrothermal post-treatment step has greatly facilitated the synthesis of MCM-48. Gemini surfactants posses two quaternary ammonium head groups separated by a methylene chain of variable length. Each head group is attached to a hydrophobic tail. Gemini surfactants have the general formula [CnH2n+1N+(CH3)2–(CH2)sN+(CH3)2CmH2m+1]·2Br, which will be abbreviated to gemini n-s-m.
The selection of a surfactant that favours the formation of MCM-48 is based on the surfactant packing parameter g ( = V/a0l), where V equals the total volume occupied by the alkyl tail group, a0 is the effective head group area at the micelle surface and l is the kinetic length of the alkyl chain.1–3 The head group area a0 changes significantly with the length of the spacer (s). At s-values below 10, the spacer is in contact with the water, lying more or less stretched at the air–water interface. At s-values above 10, the spacer becomes too hydrophobic to remain in contact with the water and folds to the air-side of the interface. Therefore, the gemini surfactant 16-12-16 with a spacer of 12 C favours the formation of the cubic MCM-48 phase, since this spacer is long enough to penetrate the hydrophobic core of the micelle.
In the conventional synthesis of a MCM-48 support, the surfactant, which makes up almost 60 wt% of the synthesis gel, is simply burnt out. This operation not only destroys the expensive surfactant, it also has negative effect on the final structure of the MCM-48 material, as the silica walls condense, causing a significant shrinkage of the unit cell.
Surfactant extraction has been attempted previously, but mainly for HMS materials.4,5 In the synthesis of HMS materials, the mesoporous structure is formed by a S0I0 interaction between the silica oligomers and neutral amines. As these interactions are relatively weak, the surfactant is extracted under very mild conditions. In the case of MCM-48 and MCM-41, the interaction between the surfactant and the silicate oligomers is an electrostatic S+I− interaction,6,7 which is generally too strong to allow an extraction by a simple organic polar solvent. Although some extraction procedures have been reported in more recent literature,4,8 the extraction was usually not very efficient and needed to be followed by a calcination step to remove the remaining surfactant. Also, the quality of the materials obtained in this way was usually inferior to the materials obtained by direct calcination.
In this paper we will discuss a method that allows the quantitative extraction of the surfactant, without a subsequent calcination step. We were able to extract the surfactant in such a way that it can be re-used several times, without degradation of the surfactant or the produced MCM-48. Finally, comparing the extracted and calcined materials, it is shown that the extracted materials have narrower pore size distributions and a larger average pore radius.
Experimental
Synthesis of materials
The Gemini surfactant is prepared by refluxing stoichiometric amounts of α,ω-dibromoalkane (s) and N,N-dimethylalkylamine (n = m) in acetone for 24 h, followed by several recrystallisations from acetone. The molar gel composition is GEM/NaOH/H2O/TEOS = 0.06/0.6/150/1. After stirring the solution for 2 h, the gel is transferred to an autoclave at 100°C for five days. The resulting white solid is obtained by vacuum filtration. The filtration cake is then repulped with 20 g of fresh water per gram of cake and the resulting suspension is returned to the autoclave at 100°C for three days. Then, the resulting product is vacuum filtered off, washed with fresh water and air dried at room temperature overnight. This material still contains the surfactant, and will be referred to as the MCM-48 precursor.Surfactant extraction was performed by refluxing 1 g of MCM-48 precursor with 50 ml of the extracting solvent. After 2 h, the white solid is filtered off, washed with fresh solvent (50 ml solvent per gram of product) and air dried overnight. The final materials were dried at 200°C prior to analysis.
The extracted surfactants were recovered by evaporation of the extracting solvent at 50°C, followed by a recrystallisation from acetone at 0°C.
Characterisation
The X-ray diffraction measurements were performed on a Philips PW 1840 powder diffractometer, using Ni-filtered Cu Kα radiation. Diffuse reflectance infrared spectra (DRIFT spectra) were recorded on a Nicolet 20 SXB FTIR spectrometer, equipped with a Spectra-Tech diffuse reflectance accessory. In situ DRIFT spectra were recorded on a Nicolet Nexus infrared bench, equipped with a catalytic chamber of Spectra Tech, allowing measurements at high vacuum and elevated temperatures. Thermogravimetric analysis was performed on a Mettler TG 50/TA 3000 thermobalance, controlled by a TC10A microprocessor. Samples were heated at a rate of 10°C min−1 under a O2-flow (150 ml min−1).Surface
area and pore volume measurements were obtained
from nitrogen adsorption isotherms at − 196°C, recorded on a
Quantachrome Autosorb-MP automated gas adsorption system.
The calcined samples were degassed at 150°C, the extracted
ones were degassed at 80°C for 16 h prior to analysis.
Surface areas were calculated according to the BET equation,
pore size distributions were calculated from the adsorption
isotherm using the newly developed KJS (Kruk–Jaroniec–Sayari)
method (![[italic v]](https://www.rsc.org/images/entities/i_char_e0f5.gif) ide infra).
ide infra).
Results and discussion
Optimisation of the extraction mixture
Several extraction media were tested for their efficiency in extracting the gemini surfactant from the MCM-48 precursor under mild reflux conditions. After each extraction, the amount of residual surfactant was determined using thermogravimetric analysis. Some of the results are presented in Table 1. It can be inferred from this table that the use of pure ethanol results in the extraction of only 16 wt% of the surfactant. This confirms the earlier made statement that polar solvents are not able to extract the cationic surfactants in a S+I− interaction with the matrix. However, the introduction of small cations (H+, NH4+, Na+) significantly improves the extraction efficiency (Table 1). This is not surprising, as the negative charges on the silicate network, which are interacting with the cationic surfactant, need to be compensated. From the several extraction media listed, acidified methanol seems the best choice: not only does it replace the surfactant by H+, resulting in the desired Si–OH groups on the surface, it also has the highest extraction efficiency. Sodium ions are not desirable, as an additional ion exchange reaction would be required to produce surface silanols; the ammonium ions can be thermally converted into protons.| Extracting medium | % Template extracted for MCM-48a |
|---|---|
| a Actual percentages of extractions are higher, due to the interference of methoxy groups, as discussed in the text. | |
| EtOH (50 ml) | 16 |
| EtOH (50 ml)/Na-acetate (0.5 g) | 64 |
| EtOH (50 ml)/NH4-acetate (0.5 g) | 68 |
| EtOH (45 ml)/HCl (5 ml, conc.) | 73 |
| Acetone (45 ml)/HCl (5 ml, conc.) | 68 |
| MeOH (45 ml)/HCl (5 ml, conc.) | 86 |
| MeOH (45 ml)/HBr (5 ml, conc.) | 85 |
| MeOH (30 ml)/HBr (20 ml, conc.) | 30 |
| MeOH (40 ml)/HBr (10 ml, conc.) | 50 |
| MeOH (45 ml)/HBr (5 ml, conc.) | 85 |
| MeOH (48 ml)/HBr (2 ml, conc.) | 62 |
| MeOH (35 ml)/HBr (5 ml, conc.)/ | |
| water (10 ml) | 57 |
The nature of the acidifying acid and the optimum ratio of methanol/acid was further investigated. Different ratios of methanol/HCl and methanol/HBr were compared, and the best results were obtained with a 9/1 vol% methanol/conc. HX mixture (X = Cl or Br). There is no significant difference between HBr and HCl as acidifiers, but as the original surfactant contains two Br ions as counter-ion, HBr is preferred in order to recover and re-use the surfactant.
Table 2 shows that a single extraction is sufficient to remove up to 86% of the surfactant. If the extracted MCM-48 is filtered, washed with pure solvent and again refluxed for 2 h with the acidified methanol, the extraction is improved by only a few percent (up to 90%), confirming that a very efficient extraction can be achieved by a single cycle.
| Samples | Wt% of surfactant removed by extraction |
|---|---|
| MCM-48 (MeOH/HCl) 2 h | 86 |
| MCM-48 (MeOH/HCl) (2 h + 2 h) | 90 |
| MCM-48 (MeOH/HBr) 2 h | 85 |
| MCM-48 (MeOH/HBr) (2 h + 2 h) | 90 |
The efficiency of the extraction is exemplified by the thermograms shown in Fig. 1, which were recorded in a flow of oxygen from 120–800°C, after preheating at 120°C for 30 min to remove all physisorbed water.
 | ||
| Fig. 1 Thermogravimetric analysis curves (——) and the differential thermogravimetric curves (– – –) of MCM-48 before (a) and after (b) extraction with MeOH + HCl. | ||
The non-extracted sample [Fig. 1(a)] typically shows a weight loss of about 50 wt%, which is due to the removal of the surfactant. It has been previously reported in literature that the oxidative decomposition of the surfactant proceeds in different steps, which explain the several minima observed in the DTG spectrum.9–11 The MCM-48 precursor that has been extracted by the acidified methanol mixture [Fig. 1(b)] hardly shows any weight loss at all (less than a few wt%).
The process of surfactant extraction was also followed by FTIR spectroscopy. Fig. 2 presents the DRIFT spectra of the MCM-48 precursor before (a) and after extraction (b) with acidified methanol.
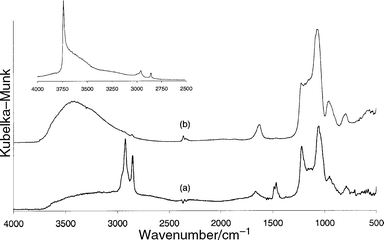 | ||
| Fig. 2 DRIFT spectra of the MCM-48 precursor before (a) and after extraction (b) with acidified MeOH. The inset represents the in situ DRIFT spectrum of a vacuum dried extracted MCM-48. | ||
The spectrum in Fig. 2(a) shows clear and distinct bands in the 2950–2850 cm−1 region, assigned to the different C–H stretching vibration of the surfactant. After extraction, these bands almost completely disappear and a large band in the 3700–3000 cm−1 region appears, due to hydrogen interacting silanols.
To establish whether these silanols are the desired isolated silanols that are typically found on a MCM-48 material, the extracted MCM-48 was vacuum dried at 120°C and an in situ DRIFT spectrum was taken (inset in Fig. 2). It shows a sharp distinct band at 3747 cm−1, typical for isolated silanol groups.
Comparison of the crystallinity and porosity of calcined and extracted materials
The physical characteristics of the extracted and calcined materials have been investigated carefully. The surface area was calculated using the BET model, while the pore diameter and the pore size distributions were calculated from the adsorption branch of the isotherms by the newly developed KJS model.2,3 Although in most studies the BJH method is used to calculate the mesoporous pore size distributions, mostly on the desorption isotherm, it is now generally accepted that this method produces large errors, especially for supra-micropores and small mesopores, with pore diameters in the region 20–60 Å. The error increases as the pore size decreases, from about 40% for a pore diameter of 20 Å, almost linearly to 0% for pore diameters of 80 Å.A lot of work is being performed to update the non-local density functional theory, which was originally calibrated using active carbon as a reference material, but no models are available at the moment for MCM-48 materials and the models that have been developed are not commercially available.
Kruk, Jaroniec and Sayari have developed the empirical KJS model, which is easily applicable due to its simplicity and validity over a broad pore region (pore diameters 20–100 Å). It is also one of the few models that allows one to differentiate between hydrophilic and hydrophobic structures. Although originally developed for honeycomb structures, extensive testing on high quality MCM-48 has satisfied us that the model can also be used for the cubic MCM-48.14
A clear difference in physical characteristics between the extracted and the calcined materials can be inferred from Table 3. The surface area and the pore volume of the extracted and calcined materials are very similar, but the pore diameter increases from 3.2 nm for the calcined sample to 3.6 nm for the extracted one. The same trend is found in XRD analysis; the cubic unit cell parameter increases form 7.9 nm for a calcined sample to 8.5 nm for the extracted one.
| Sample | Unit cell, a/nm | Pore diameter, D/nm | SBET/ m2 g−1 | Pore volume/ cm3 g−1 | Wall thickness/ nm |
|---|---|---|---|---|---|
| MCM-48 calcined | 7.92 | 3.2 | 1464 | 1.85 | 0.96 |
| MCM-48 extracted | 8.54 | 3.6 | 1331 | 1.76 | 0.96 |
| with MeOH and HCl | |||||
The wall thickness given in Table 3 was calculated from adsorption data and XRD data using a recently developed theory by Schumacher,15 resulting in an easily applicable formula: 〈h〉 = (a/3.092) − (Dh/2), where Dh is the mean pore diameter calculated by the KSJ model and a is the unit cell parameter as calculated from the XRD patterns.
There are no differences in the pore wall thickness between the extracted and the calcined samples, both samples having a typical pore wall thickness of about 1 nm.
To gain further insight into the structural differences between the two materials, the KJS pore size distributions are shown in Fig. 3. The pore size distribution (PSD) of the extracted sample (b) is narrower than the PSD of the calcined sample (a). Moreover, as could already be read from Table 3, the extracted materials have clearly larger pores. Both phenomena can be explained by assuming that the oxidative decomposition of the surfactant during calcination causes very high local temperatures, resulting in a slight structural collapse, resulting in a broader pore size distribution. Moreover, the high temperature of calcination initiates condensation of silanols and shrinkage of the unit cell. Because of the soft extraction procedure, no unit cell shrinkage occurs during extraction, resulting in a very narrow pore size distribution with larger pores.
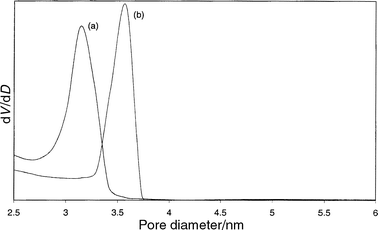 | ||
| Fig. 3 Comparison of the KSJ pore size distribution curves of MCM-48 calcined (a) and MCM-48 extracted with MeOH + HCl (b). | ||
Determination of the silanol number
The exact amount of silanol group on an MCM-48 surface has not yet been determined accurately in the literature. Knowledge of the exact concentration of silanol groups is very important, however, to estimate the adsorption capacity and the maximum number of catalytic sites that can be grafted onto the surface.In the mid-1990s we developed a very convenient method to quantitatively assess the silanol number.16 The principles are based on the reaction of a silica surface with HMDS (hexamethyldisilazane), followed by a titration of liberated NH3, which is captured in a solution of boric acid.
The results, obtained by the HMDS method are listed in Table 4.
| Sample | SBET/m2 g−1 | No. OH nm−2 |
|---|---|---|
| Calcined MCM-48 | 1412 | 0.9 |
| Extracted MCM-48 | 1287 | 0.75 |
It can be inferred from Table 4, that a blank, calcined MCM-48, prepared by the gemini 16-12-16 surfactant, and calcined at 550°C has a silanol number of 0.9 ± 0.05 OH nm−2 as measured after vacuum drying at 200°C. These results correspond well with the theoretical considerations of Alami et al.,17 who calculated that the ‘head group area’ for a gemini surfactant with a spacer of 12 C atoms amounts to 2.26 nm2. Each headgroup participates in 2Si–O− − NR4+ functions during the synthesis of the MCM, which are converted into Si–OH functions upon calcination. The theoretical silanol density of a MCM-48, prepared by the 16-12-16 gemini surfactant and a 100% silica source is therefore 2 OH per 2.26 nm2 or 0.9 OH nm−2.
The extracted MCM-48 samples have slightly fewer silanols on the surface (0.75 ± 0.05 OH nm−2). As the difference in silanol number is too high to be accounted for completely by inefficient extraction, we looked for other possible surface groups on the MCM-48 materials.
Fig. 4(b) shows only the (strongly enlarged) alkyl region of the DRIFT spectrum of the extracted material. The bands at 2850, 2925 and 1430 cm−1 are typical vibrations of the residual surfactant, as is obvious from the comparison with spectrum (a), which is the DRIFT spectrum of the precursor (MCM-48 with the surfactant still in it). The band at 2967 cm−1, however, cannot be assigned to residual surfactant, as this is a methoxy vibration. These methoxy groups are apparently created on the surface during the extraction of the surfactant in acidified methanol. We have already proven in a previous publication18 that the surface of MCM-48 is much more reactive than the surface of amorphous silica gel. To verify whether acidified methanol is able to produce methoxy groups on the MCM-48 surface, we refluxed ordinary calcined MCM-48 with the acidified methanol. In this case, the surfactant was already removed by normal calcination, before the MCM-48 came into contact with the acidified methanol.
 | ||
| Fig. 4 DRIFT spectra of (a) MCM-48 precursor containing the gemini surfactant; (b) MCM-48 extracted with acidified MeOH; (c) MCM-48 calcined and reacted with acidified MeOH. | ||
The resulting infrared spectrum is shown as spectrum (c) in Fig. 4. It shows the significant band around 2967 cm−1, confirming the reaction of acidified methanol with the surface of MCM-48. The number of surface methoxy groups can then be estimated as ca. 0.15 groups nm−2 on the extracted samples. We expect that the presence of some methoxy groups on the surface will render the material slightly more hydrophobic, which is beneficial in several liquid phase catalytic reactions.19,20
Recuperation and re-use of the extracted surfactant
It is not only important that the quality of the extracted samples is equal to or better than the quality of the calcined samples, the extracted surfactant should be recovered without loss of quality and should be re-used several times for new syntheses.The extracted surfactant was therefore recrystallised from acetone and analysed. Elemental analysis shows that the extracted surfactant does not degenerate during the extraction. The chemical ratio C/N/Br was found to be 48/2/2 for the original surfactant, 46/2/2 for a surfactant extracted with MeOH/HBr and 46/2/0.5 for a surfactant extracted with MeOH/HCl. In the latter case, Cl anions replaced the Br anions as counter-ions.
Also, DRIFT and XRD analysis [Fig. 5(A) and 5(B), respectively] clearly indicate that the recuperated surfactant has the same functionalities as and a similar crystallographic structure to the original surfactant.
 | ||
| Fig. 5 Comparison between the DRIFT spectra (A) and the X-ray diffraction patterns (B) of the parent gemini surfactant (a) and the recovered gemini surfactant after extraction (b). | ||
The reproducibility of the synthesis of MCM-48, and the quality of the resulting materials, using the extracted and recovered surfactant, was also evaluated. The recovered gemini surfactant was used to prepare MCM-48, following the same method of synthesis of MCM-48 prepared using pure gemini surfactant.
Fig. 6 compares the nitrogen adsorption isotherms (A) and the pore size distributions (B) of the MCM-48 synthesised using the parent gemini surfactant (a) and the recovered gemini surfactant (b).
 | ||
| Fig. 6 Comparison between the N2 adsorption isotherms (A) and the KJS pore size distributions (B) of MCM-48 synthesised using the parent gemini surfactant (a) and the recovered gemini surfactant (b). Both materials have been calcined to remove the surfactant. | ||
It is obvious from Fig. 6 that the use of recovered gemini surfactant has no effect on the quality of porosity of the final materials. This was also confirmed by XRD results, which showed no significant differences between the diffractograms of the two samples.
Conclusion
Acidified methanol extracts more than 90% of the surfactant from a MCM-48 precursor. The resulting material has an even narrower pore size distribution and larger pores than MCM-48 prepared by a regular calcination of the surfactant.The silanol number of the extracted and calcined MCM-48 have been determined experimentally and compared. A calcined sample contains 0.9 OH nm−2, a surfactant extracted sample contains 0.75 OH nm−2. This lower amount of silanols is due to the replacement of some of the silanols by methoxy groups.
The extracted surfactant does not degenerate and has the same physical and chemical characteristics as the parent surfactant. It can be re-used easily for subsequent syntheses. MCM-48 materials prepared by recovered surfactant show no reduction in porosity, crystallinity or quality. In this way, the extraction and re-use of the gemini surfactant is a major step forward, both ecologically and economically, as the expensive starting materials can be re-used efficiently.
Acknowledgements
PVDV and PC acknowledge the FWO Vlaanderen (Fund for Scientific Research–Flanders–Belgium) for financial support.References
- Q. Huo, R. Leon, P. M. Petroff and G. D. Stucky, Science, 1995, 268, 1324 CrossRef CAS.
- Q. Huo, D. E. Margolese and G. D. Stucky, Chem. Mater., 1996, 8, 1147 CrossRef CAS.
- P. Van Der Voort, M. Mathieu, F. Mees and E. F. Vansant, J. Phys. Chem. B., 1998, 102, 8847 CrossRef CAS.
- R. Mokaya and W. Jones, J. Mater. Chem., 1998, 8, 2819 RSC.
- P. T. Tanev and T. J. Pinnavaia, Chem. Mater., 1996, 8, 2068 CrossRef CAS.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865 CrossRef CAS.
- S. A. Bagshaw, E. Prouzet and T. J. Pinnavaia, Science, 1995, 269, 1242 CrossRef.
- S. Hitz and R. Prins, J. Catal., 1995, 168, 194 CrossRef CAS.
- N. K. Raman, M. T. Anderson and C. J. Brinker, Chem. Mater., 1996, 8, 1682 CrossRef CAS.
- C. Y. Chen, H. X. Li and M. E. Davis, Microporous Mater., 1993, 2, 17 CrossRef CAS.
- X. S. Zhao, G. Q. Lu, A. K. Whittaker, G. J. Millar and H. Y. Zhu, J. Phys. Chem. B, 1997, 101, 6525 CrossRef CAS.
- M. Jaroniec, M. Kruk and A. Sayari, Stud. Surf. Sci. Catal., 2000, 129, 587 Search PubMed.
- M. Kruk, M. Jaroniec and A. Sayari, Langmuir, 1997, 13, 6267 CrossRef CAS.
- P. Van Der Voort and M. Jaroniec, unpublished results..
- K. Schumacher, P. I. Ravikovitch, A. Du Chesne, A. V. Neimark and K. K. Unger, Langmuir, 2000, 16, 4648 CrossRef CAS.
- E. F. Vansant, P. Van Der Voort and K. C. Vrancken, Characterization and Modification of the Silica Surface, in Studies in Surface Science and Catalysis, Elsevier Science Publishers, Amsterdam, 1995, vol. 93. Search PubMed.
- E. Alami, G. Beinert, P. Marie and R. Zana, Langmuir, 1993, 9, 1465 CrossRef CAS.
- M. Baltes, K. Cassiers, P. Van Der Voort, B. M. Weckhuysen, R. A. Schoonheydt and E. F. Vansant, J. Catal., in press. Search PubMed.
- A. Hagen, D. Wei and G. Haller, Stud. Surf. Sci. Catal., 1998, 117, 191 Search PubMed.
- A. Corma and D. Kumar, Stud. Surf. Sci. Catal., 1998, 117, 201 Search PubMed.
| This journal is © the Owner Societies 2001 |