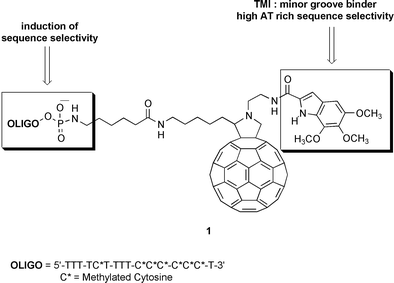
Synthesis of a hybrid fullerene–trimethoxyindole–oligonucleotide conjugate
Massimo
Bergamin
a,
Tatiana
Da Ros
a,
Giampiero
Spalluto
a,
Alexandre
Boutorine
b and
Maurizio
Prato
*a
aDipartimento di Scienze Farmaceutiche, Università di Trieste, Piazzale Europa 1, 34127, Trieste, Italy. E-mail: prato@univ.trieste.it
bLaboratoire de Biophysique, INSERM U 201 - CNRS UMR 8646, Museum National d’Histoire Naturelle, 43 rue Cuvier, 75231, Paris, Cedex 05, France
First published on 11th December 2000
Abstract
The synthesis of novel functionalized fullerene derivatives is reported: a DNA minor groove binder such as trimethoxy- indole-2-carboxylate (TMI) and an oligonucleotide chain have been covalently linked to C60 with the aim of duplicating DNA interactions for increasing sequence selectivity.
Fullerene C60 and its organofunctionalized derivatives have recently become a topic of interest in bioorganic and medicinal chemistry. This class of compounds has shown high potential in a wide variety of biological activities, such as DNA photocleavage, HIV protease inhibition, neuroprotection and apoptosis.1 In particular, the excited state properties of C60 may offer viable routes to novel pharmacological tools.2
One of the problems of photodynamic therapy is the addressed delivery of a photoactive agent to its target. That is why conjugates of fullerene with molecules possessing biological affinity to certain nucleic acids, proteins, cell types, organelles, etc., might be of particular interest. Additionally, C60 itself might facilitate the interactions of certain biologically active molecules with lipophilic membranes of living cells and consequently improve cellular uptake due to its high hydrophobicity.
Recently, some conjugates between C60 and nucleic acid-specific agents (acridine,3 netropsin4 and complementary oligonucleotides5,6) have been reported, with the aim of better understanding the mechanism of action and to increase their cytotoxic properties and specificity. Coupling of fullerene to an intercalator or a minor groove binder already leads to higher affinity and specificity of the derivatives towards target DNA.4 The next step envisages the improvement of the photocleavage efficiency of the conjugates, possibly by stabilizing their duplexes and triplexes and increasing their sequence selectivity.
In order to achieve these goals, we have designed derivative (1) of fulleropyrrolidine linked to trimethoxyindole (TMI) and an oligonucleotide chain simultaneously. The rational design of this derivative was based on a synergistic effect of two different linkers such as TMI and oligonucleotide. The TMI unit is characteristic of a class of natural antibiotics named duocarmycins, possessing antitumor activity in the picomolar range, in part due to high affinity and selectivity of the TMI group for the minor groove in AT-rich sequences of DNA.7 The effect of the oligonucleotide chain could be considered both to induce high sequence-selectivity and to increase water solubility, one of the main problems of C60 derivatives for biological application.
The designed compound 1 was prepared following the general synthetic strategy summarized in Schemes 1–2, according to the known procedure for the synthesis of fulleropyrrolidines, based on the 1,3-dipolar cycloaddition of azomethine ylides to C60.8,9
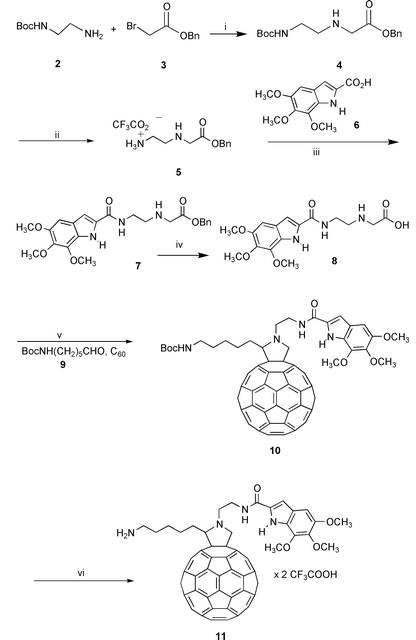 | ||
| Scheme 1 Reagents: i, dioxane, 0 °C, then rt 18 h; ii, TFA, CH2Cl2, rt, 20 min; iii, NMM, HOBT, EDC, CH2Cl2; iv, Pd/C 10%, H2, MeOH; v, toluene, reflux, 50 min; vi, TFA, CH2Cl2, rt, 1 h. | ||
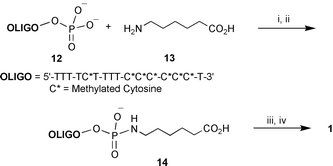 | ||
| Scheme 2 Reagents: i, PPh3, DMSO, DMAP, Py2S2; ii, DMSO; iii, PPh3, DMSO, Py2S2; iv, Et3N, DMSO, 11. | ||
The appropriate O-benzylaminoacid 4 was prepared by alkylation of the mono protected (Boc) ethylenediamine 2 with benzyl α-bromoacetate 3 (Scheme 1).
Deprotection of the amino functionality by TFA in 8 led to amine 5, which was coupled to trimethoxyindole-2-carboxylic acid 610 to afford amino ester 7. The latter was deprotected at the carboxylic function by catalytic hydrogenation and the resulting acid 8 was allowed to react with C60 and N-Boc-6-aminohexanal 9 to yield the desired multifunctional fulleropyrrolidine 10.† The latter compound was in turn deprotected to obtain the desired salt 11 by treatment with TFA (Scheme 1).
The designed product 1 was synthesized following a general synthetic strategy for the preparation of oligonucleotide conjugates previously reported11–13 and summarized in Scheme 2, but the new procedure contained several modifications. Originally the reaction was initiated by activation of the oligonucleotide terminal phosphate by means of Mukaiyama reagents, triphenylphosphine–dipyridyl-2,2′-disulfide in the presence of DMAP.14 However, it appeared that interaction of the activated phosphate with the amino group of the fullerene derivative did not lead to satisfactory yields of conjugation. Thus, it was necessary to use 6-aminocaproic acid as a spacer between the two moieties.
The phosphorylated oligonucleotide (16-mer) 12 was activated at its terminal phosphate, precipitated by lithium perchlorate in acetone according to the method described by Knorre et al.11 and attached to the ε-amino group of 6-aminocaproic acid 13 in water in the presence of triethylamine to afford the carboxylic acid derivative of oligonucleotide 14 in quantitative yield. Coupling of 14 with fullerene derivative 11 was performed in a similar way, by activation of the carboxylic group with Mukaiyama reagents in organic media to obtain the desired conjugate 1. Purification of 1 was performed by electrophoresis in 1% agarose–0.1% triton X-100 gel using tris-acetate buffer, followed by excision of the colored band and electroelution, or digestion of agarose with β-agarase.5,6
In summary, we have described the synthesis of the bis-functionalized fulleropyrrolidine 1 containing two different linkers capable of modulating and inducing high sequence selectivity. Results on duplex and triplex helicex DNA formation and the synthesis of an enlarged series of compounds for structure–activity relationship studies will be reported in due course.
Acknowledgements
This work was supported by MURST (cofin. ex 40%, prot. n. mm03198284), by Regione Friuli-Venezia Giulia, fondo Anno 1998, and by the European Community (TMR program BIOFULLERENES, contract No. ERB FMRX-CT98-0192).Notes and references
- T. Da Ros and M. Prato, Chem. Commun., 1999, 663 RSC.
- D. Guldi and M. Prato, Acc. Chem. Res., 2000, 33, 695 CrossRef CAS.
- Y. N. Yamakoshi, T. Yagami, S. Sueyoshi and N. Miyata, J. Org. Chem., 1996, 61, 7236 CrossRef.
- E. Nakamura, H. Tokuyama, S. Yamago, T. Shiraki and Y. Sugiura, Bull. Chem. Soc. Jpn., 1996, 69, 2143 CAS.
- A. Boutorine, H. Tokuyama, M. Takasugi, H. Isobe, E. Nakamura and C. Hélène, Angew. Chem., Int. Ed. Engl., 1994, 33, 2462 CrossRef.
- Y.-Z. An, C.-H. B. Chen, J. L. Anderson, D. S. Sigman, C. S. Foote and Y. Rubin, Tetrahedron, 1996, 52, 5179 CrossRef CAS.
- D. L. Boger and D. S. Johnson, Angew. Chem., Int. Ed. Engl., 1996, 35, 1438 CrossRef CAS.
- M. Maggini, G. Scorrano and M. Prato, J. Am. Chem. Soc., 1993, 115, 9798 CrossRef CAS.
- M. Prato and M. Maggini, Acc. Chem. Res., 1998, 31, 519 CrossRef CAS.
- D. Boger, T. Ishizaki, H. Zarrinmayeh, P. Kitos and O. Suntornwat, J. Org. Chem., 1990, 55, 4499 CrossRef CAS.
- D. G. Knorre, P. V. Alekseyev, Y. V. Gerassimova, V. N. Silnikov, G. A. Maksakova and T. S. Godovikova, Nucleosides, Nucleotides, 1998, 17, 397 Search PubMed.
- T. S. Godovikova, V. F. Zarytova, T. V. Maltseva and L. M. Khalimskaya, Bioorg. Khim., 1989, 15, 1246 Search PubMed.
- A. S. Boutorine, T. Le Doan, J. P. Battioni, D. Mansuy, D. Dupré and C. Hélène, Bioconj. Chem., 1990, 1, 350 CrossRef CAS.
- T. Mukaiyama, R. Matsueda and M. Suzuki, Tetrahedron Lett., 1970, 22, 1901 CrossRef CAS.
Footnote |
| † Analytical and spectroscopic data of derivative 10: C86H40N4O6 (MW 1225.30), yield: 19%. 1H-NMR: δ 9.27 (br s, 1H), 7.30 (br s, 1H), 6.93 (s, 1H), 6.75 (s, 1H), 4.98 (d, J = 10.3 Hz, 1H), 4.52 (m, 1H), 4.37 (t, J = 5.9 Hz, 1H), 4.30 (d, J = 10.5 Hz 1H), 4.08–3.95 (m, 4H), 4.05 (s, 3H), 3.89 (s, 3H), 3.85 (s, 3H), 3.25 (m, 1H), 3.11 (m, 2H), 2.45 (m, 2H), 1.89 (m, 2H), 1.49 (m, 2H), 1.43 (s, 9H). 13C-NMR: δ 166.9, 156.4, 154.4, 153.2, 150.0, 147.1, 146.3, 146.2, 146.1, 146.0, 145.9, 145.5, 145.2, 145.1, 144.6, 144.4, 144.3, 143.0, 142.6, 142.2, 142.1, 142.0, 141.9, 141.7, 140.2, 139.7, 136.7, 135.8, 135.6, 135.4, 130.6, 123.3, 97.1, 71.2, 67.4, 65.4, 61.1, 56.2, 44.1, 41.1, 31.0, 29.9, 28.5. ES-MS (THF–MeOH 1∶1): m/z 1225 (MH+). UV–VIS (cyclohexane) λmax: nm 254, 312, 431, 703. IR (KBr, DRIFT) cm−1: 3330, 2935, 2850, 1690, 1645, 1245, 1169, 526. |
| This journal is © The Royal Society of Chemistry 2001 |