Asymmetric cyclopropanation in protic media conducted by chiral bis(hydroxymethyl-dihydrooxazolyl)pyridine–ruthenium catalysts
Seiji Iwasa, Futoshi Takezawa, Yasunori Tuchiya and Hisao Nishiyama*
School of Materials Science, Toyohashi University of Technology, Tempaku-cho, Toyohashi 441-8580, Japan.. E-mail: hnishi@tutms.tut.ac.jp
First published on 14th December 2000
Abstract
Cyclopropanation of styrene with diazoacetates, performed in aqueous/organic biphasic media or homogeneous alcoholic media in the combination of toluene by using chiral bis(hydroxymethyldihydrooxazolyl)pyridine–ruthenium catalyst, resulted in high enantiomeric excess up to 96–97% and trans∶cis stereoselectivity to 97∶3.
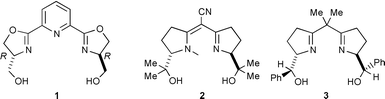
Enantioselective reactions of olefins and diazoacetates catalyzed by a variety of metal complexes to provide chiral cyclopropane materials have been well investigated.1 We have reported ruthenium catalysts of chiral bis(dihydrooxazolyl)pyridine [pybox] for that purpose and their prominent feature of high trans-stereoselectivity with higher enantioselectivity.2,3 Very recently, we reported a characteristic derivative of pybox, hm-pybox 1, having two hydroxymethyl groups as the symmetric chiral stems of the oxazoline rings.4 We observed that hm-pybox exhibits high solubility in water. One of the recent demands for organic synthesis and catalysis, with environmental concerns in mind, has been for the reactions to be carried out in non-halogenated solvents or in aqueous and protic media.5 We therefore had expectations of developing a new process in aqueous media for asymmetric catalytic cyclopropanation using our water-soluble bis(hydroxymethyldihydrooxazolyl)pyridine–ruthenium catalyst. In addition, however, we demonstrated the tolerance of the catalysis to protic media, such as alcoholic mixtures.
We surveyed previous research related to the catalytic cyclopropanation of olefins and diazoacetates but we could find no systems effective in aqueous media or protic solvents.4 However, we discovered that the existence of a free hydroxy group on chiral ligands does not interfere with the smooth running of cyclopropanation for copper catalyzed reactions, for example, in the case of bis(oxazoline) ligands 26 or 37. It had also very recently been reported that although a small amount of water in the reaction solvent diminishes enatioselectivity of cyclopropanation with rhodium catalyst, the unfavorable effect of water was reduced by addition of an appropriate phosphite ligand.8 Accordingly, we were intrigued to examine the catalysis with hm-pybox 1 and [RuCl2(p-cymene)]24.
First, we tried an aqueous media for the cyclopropanation of styrene and (+)-menthyl diazoacetate 5a with hm-pybox in the presence of co-solvent THF or toluene (Scheme 1). (+)-Menthyl ester was chosen on the basis of better matching to the (R,R)-absolute configuration of pybox, which ought to give higher enantioselectivity according to our previous work.2b The use of a single organic solvent resulted in lower yields and lower enantioselectivities (run 1 and 2 in Table 1). Surprisingly, addition of water to both media in runs 1 and 2 dramatically improved the enantioselectivities and slightly the yields (runs 4 and 5). This phenomenon can be simply accounted for by the increase of the solubility of the active catalyst Ru(hm-pybox)Cl2(vacant or solvent) derived from hm-pybox 1 and pre-catalyst [RuCl2(p-cymene)]24. It could easily be seen from the dark-violet coloring of the bottom phase that most of the catalyst was dissolved in the aqueous phase. Into the two-phase system of water and organic solvent (initial ratio = 1∶2), a solution of the diazoacetate 5 was slowly added under vigorous stirring to give the desired cyclopropanes 6 in moderate yields with higher enantioselectivity (88% for 6t, runs 3 and 4). The organic layer was extracted with degassed (or absolute) diethyl ether and concentrated to give the products. As the active species remained in the aqueous phase, the second run was carried out by addition of styrene and diazoacetate to give a similar result (run 5). We are now further investigating the optimization and multi-time reuse of the catalyst.
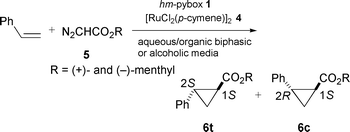 | ||
| Scheme 1 | ||
| 6t + 6cb | %Eec | |||||
|---|---|---|---|---|---|---|
| Solvent of | ||||||
| Run | Initial solvent (ml) | 5a (ml) | Yield % | Ratio | 6t | 6c |
| a Styrene (10 mmol), diazoacetate (2.0 mmol), pybox (0.14 mmol), [RuCl2(p-cymene)]2 (0.05 mmol, 5 mol% of Ru), 40 °C. A solution of diazoacetate in 3.0 ml of the same solvent was slowly added by syringe for 6 h to the mixture of styrene and the catalyst in the initial solvent.b Isolated yield, ratios by 1H NMR.c %Ee determined by the reported method, see ref 2. Absolute configuration: 6t for all runs, (1S,2S); 6c for run 1 and 2, (1R,2S); 6c for runs 3 and 4, (1S,2R).d Half scale for run 1: diazoacetate (1.0 mmol), styrene (5.0 mmol), catalyst 5 mol%, for 4 h. e After ether extraction of the reaction mixture of the first run, styrene (5.0 mmol) and toluene (1.5 ml) were added followed by slow addition of 5a in toluene. | ||||||
| 1 | THF (3) | THF (3) | 39 | 83∶17 | 8 | 30 |
| 2 | Toluene (3) | Toluene (3) | 38 | 89∶11 | 8 | 28 |
| 3 | THF (2) + H2O (1) | THF (3) | 46 | 95∶5 | 78 | 45 |
| 4 | Toluene (2) + H2O (1) | Toluene (3) | 56 | 96∶4 | 88 | 51 |
| 51std | Toluene (0.5) + H2O (0.5) | Toluene (1.5) | 57 | 97∶3 | 94 | 76 |
| 52nde | Toluene (1.5) | 62 | 97∶3 | 97 | 90 | |
In this aqueous system, addition of phase-transfer reagents such as (n-Bu4N)(HSO4) (10 mol% of 5a) into the system of run 4 resulted in no improvement upon the reaction and the selectivities. On the other hand, when alcohols such as ethanol, isopropyl alcohol, and tert-butyl alcohol in place of water were adopted to provide a homogeneous protic media, isopropyl alcohol resulted in the best enantioselectivities, up to 96% ee for trans-6t and 88% ee for cis-6c at 30 °C (Table 2, run 4). (−)-Menthyl diazoacetate 5b showed a decrease of ee to 90% for trans-product (run 5), because of the unmatched steric pair toward R,R-absolute configuration of the ligand. Single use of isopropyl alcohol gave moderate ees (run 7). We have thus found that the choice of alcoholic solvents apparently influences the enantioselectivity. However, at present we cannot define the origin of the stereochemical outcomes for protic solvents.
| 6t + 6c | %Eeb | |||||
|---|---|---|---|---|---|---|
| Solvent of | ||||||
| Run | Initial solvent (ml) | 5a (ml) | Yield % | Ratio | 6t | 6c |
| a The reaction scale and procedures were the same as those described in Table 1.b Absolute configuration: 6t for all runs, (1S,2S); 6c for run 2 and 6, (1R,2S); 6c for runs 1,3,4,5 and 7, (1S,2R). c At 30 °C. The cyclopropanation did not proceed at 20 °C. d In place of (+)-menthyl diazoacetate, (−)-menthyl ester 5b was used. | ||||||
| 1 | Toluene (1) + EtOH (1) | Toluene (3) | 67 | 96∶4 | 35 | 2 |
| 2 | Toluene (1) + t-BuOH (1) | Toluene (3) | 54 | 91∶9 | 11 | 15 |
| 3 | Toluene (1) + i-PrOH (1) | Toluene (3) | 78 | 95∶5 | 92 | 65 |
| 4c | Toluene (1) + i-PrOH (1) | Toluene (3) | 52 | 97∶3 | 96 | 88 |
| 5d | Toluene (1) + i-PrOH (1) | Toluene (3) | 78 | 95∶5 | 90 | 88 |
| 6 | THF (1) + i-PrOH (1) | THF (3) | 73 | 96∶4 | 89 | 58 |
| 7 | i-PrOH (2) | i-PrOH (3) | 59 | 95∶5 | 84 | 30 |
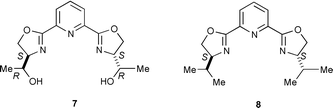
We next intended stereochemical tuning of the hydroxymethyl group of 1 using hydroxyethyl-pybox 7 [he-pybox] synthesized from (−)-threonine.† However, in aqueous biphasic media, toluene–H2O, the enantioselectivities with ruthenium–7 catalyst were not increased by using (−)-menthyl diazoacetate at 40 °C: 80% ee for trans and 50% ee for cis, in 96∶4 trans∶cis ratio (75% yield). In toluene–i-PrOH the enantioselectivities increased to 91% for trans and 78% ee for cis, in 94∶6 trans∶cis ratio (78% yield). In comparison, classic ip-pybox 8 with similar bulkiness to 7 was found in toluene–i-PrOH media to give 93% ee for trans and 90% ee for cis, in 97∶3 trans∶cis ratio (84% yield). He-pybox 7 thus proved to be inferior to iso-pybox 8.
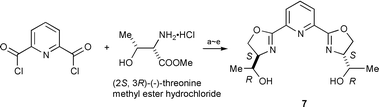 | ||
| Scheme 2 Reagents and conditions: (a) Et3N, CHCl3, rt, 12 h, 92%. (b) TBDMSCl, imidazole, CH2Cl2, rt, 3.5 h, 98%. (c) LiBH4, THF, 0 °C ∼ rt, 6 h, 75%. (d) PPh3, imidazole, CCl4, CH2Cl2, rt, 4.5 h, 44%. (e) Bu4NF (1.0 M in THF), rt, 3 h, 100%. | ||
In conclusion, the hydroxymethyl derivative of pybox can provide excellent stereoselectivities for cyclopropanation of styrene, compared to the hydroxyethyl or isopropyl derivatives, in moderate yields in aqueous and protic media. We hypothesize that appropriate solvation of water or alcohols around the hydroxy group causes a more favourable stereochemical environment around the active site for the cyclopropanation. Work is now under way to investgate the mechanism of reaction and on applications to other catalytic reactions performed in aqueous media.
Footnotes and references
References
- General reviews: Catalytic Asymmetric Synthesis 2nd, ed. I. Ojima, Wiley-VCH, New York, 2000; Search PubMed; Comprehensive Asymmetric Catalysis I-III, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto (eds.), Springer-Verlag, Berlin, 1999. Search PubMedFor catalytic cyclopropanation: M. P. Doyle, M. A. McKervey and T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, John Wiley & Sons, Inc., 1997; Search PubMed; M. P. Doyle and M. N. Protopopova, Tetrahedron, 1998, 54, 7919 Search PubMed.
- (a) (a) H. Nishiyama, Y. Itoh, H. Matsumoto, S.-B. Park and K. Itoh, J. Am. Chem. Soc., 1994, 116, 2223 CrossRef; (b) H. Nishiyama, Y. Itoh, Y. Sugawara, H. Matsumoto, S.-B. Park and K. Itoh, Bull. Chem. Soc. Jpn., 1995, 68, 1247 CAS; (c) H. Nishiyama, N. Soeda, T. Naito and Y. Motoyama, Tetrahedron: Asymmetry, 1998, 9, 2865 CrossRef CAS.
- Related researches: review for chemistry of oxazoline ligands: K. Ghosh, P. Mathivanan and J. Cappiello, Tetrahedron: Asymmetry, 1998, 9, 1 CrossRef CAS; F. Fache, E. Schulz, M. L. Tommasino and M. Lemaire, Chem. Rev., 2000, 100, 2159 CrossRef CAS.
- S. Iwasa, H. Nakamura and H. Nishiyama, Heterocycles, 2000, 52, 939 Search PubMed.
- Reviews: P. A. Grieco, Organic Synthesis in Water, Thomson Science, London, 1998; Search PubMed; B. Cornils and W. A. Herrmann, Aqueous-Phase Organometallic Catalysis, Wiley-VCH, Weinheim, 1998 Search PubMed; D. Sinou, Transition Metals for Organic Synthesis, Vol. 2, ed. M. Beller and C. Bolm, Wiley-VCH, Weinheim, 1998, 398. Search PubMedPapers for examples: K. Yonehara, T. Hashizume, K. Mori, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 5593 Search PubMed; J. Holz, D. Heller, R. Stürmer and A. Börner, Tetrahedron Lett., 1999, 40, 7059 Search PubMed; S. Kobayashi, S. Nagayama and T. Busujima, Tetrahedron, 1999, 55, 8739 Search PubMed; W. Xie, J. Fang, J. Li and P. G. Wang, Tetrahedron, 1999, 55, 12929 and references therein. CrossRef CAS.
- H. Fritchi, U. Leutenegger and A. Pfaltz, Helv. Chim. Acta, 1988, 71, 1553 CrossRef CAS.
- O. Hoarau, H. Ait-Haddou, M. Castro and G. G. A. Balavoine, Tetrahedron: Asymmetry, 1997, 8, 3775 CrossRef CAS; O. Hoarau, H. Ait-Haddou, J.-C. Daran, D. Cramailère and G. G. A. Balavoine, Organometallics, 1999, 18, 4718 CrossRef CAS.
- D. C. Wynne, M. M. Olmstead and P. G. Jessop, J. Am. Chem. Soc., 2000, 122, 7638 and references for solvent effect in catalytic cyclopropanation cited therein. Search PubMed.
Footnote |
| † Synthesis of he-pybox: the route is illustrated (Scheme 2) starting from (−)-threonine methyl ester·HCl and 2,6-pyridinecarboxylic acid chloride. 7; white solid. mp 94–95 °C. δH (300 MHz, CDCl3, Me4Si) 1.30 (d, J 6.4, 6 H), 2.70 (br d, 2 H), 3.77 (dq, 2 H), 4.29 (dt, 6 H), 4.59 (dd, 2 H), 7.91 (t, 1 H), 8.15 (d, 2 H). δC (75.5 MHz, CDCl3, Me4Si) 19.5, 70.1, 70.4, 73.2, 126.1, 137.7, 146.5, 163.6. |
| This journal is © The Royal Society of Chemistry 2001 |