Palladacyclic phosphinite complexes as extremely high activity catalysts in the Suzuki reaction
Robin B.
Bedford
* and
Samantha L.
Welch
School of Chemistry, University of Exeter, Exeter, UK EX4 4QD. E-mail: R.Bedford@ex.ac.uk
First published on 18th December 2000
Abstract
Phosphinite based palladacycles show extremely high activity in the Suzuki coupling of both sterically hindered and electronically deactivated aryl bromides, especially in the presence of one equivalent of free ligand.
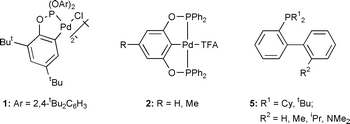
There has recently been considerable interest in the synthesis of new, high activity palladium-based catalysts that can be used in low concentration in the Suzuki reaction (Scheme 1) since such catalysts have the potential to be used in industrial systems. In particular, palladacyclic catalysts in which a ligand coordinates to the metal centre through both a donor atom and metallated carbon have shown considerable promise. Beller and co-workers demonstrated that palladated phosphine complexes show good activity1 whilst we have shown that the palladated triaryl phosphite complex 1 and the bis(phosphinite) PCP-pincer complexes 2 show excellent activity.2,3 High activity is not limited to metallated phosphorus donor systems—Milstein and co-workers have shown that a palladated imine complex shows excellent activity,4 whilst Zim et al. have shown that palladated thioether complexes can also be used.5
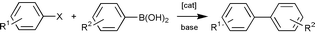 | ||
| Scheme 1 The Suzuki biaryl coupling reaction. | ||
To the best of our knowledge, the use of P,C-bidentate phosphinite palladacycles as catalysts in the Suzuki reaction has not been investigated. We report here the synthesis of such complexes, the remarkable activity they show in the Suzuki reaction and present preliminary evidence that suggests that the active catalysts are likely to be low coordinate palladium(0) species.
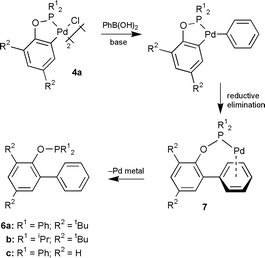
The reactions of the ligands 3a and b with palladium chloride in toluene at reflux temperature gives the complexes 4a and b in good yields. Complex 4c is best prepared by the reaction of [PdCl2(NCPh)2] with 3c in THF at reflux temperature. All the complexes 7 are obtained as a mixture of cis and trrans isomers.2
![(i) Et3N, toluene, reflux temperature, 18–20 h; (ii)
For 4a and b: PdCl2, toluene, reflux
temperature 18–20 h; (iii) For 4c:
[PdCl2(NCPh)2], THF, reflux temperature, 20 h.](/image/article/2001/CC/b008470k/b008470k-s2.gif) | ||
| Scheme 2 (i) Et3N, toluene, reflux temperature, 18–20 h; (ii) For 4a and b: PdCl2, toluene, reflux temperature 18–20 h; (iii) For 4c: [PdCl2(NCPh)2], THF, reflux temperature, 20 h. | ||
The application of the complexes 4a–c as catalysts in the Suzuki reaction was studied and the results are summarised in Table 1. The complexes 4a and b prove to be extremely active catalysts for both the ‘easy to couple’ substrate 4-bromoacetophenone and the more challenging, electronically deactivated substrate 4-bromoanisole. To the best of our knowledge, the activity shown by the catalyst mixture of 4b and one equivalent of added ligand 3b (entry 13) is the highest reported for any Suzuki reaction—over five times higher than for the analogous reaction catalysed by palladium complexes of the phosphine ligands 5.6 By contrast, the previous highest reported activity with a palladacyclic catalyst in this reaction was with complex 1 which gave a turn-over number (TON) of 1 million.2 More importantly with the electronically deactivated substrate 4-bromoanisole the catalyst systems reported here show up to 45 times higher activity than the previous best catalysts—the related bis(phosphinite) pincer complexes 2.3 Similarly the activities seen with the sterically hindered, electronically deactivated substrates 2-bromotoluene and 2-bromo-m-xylene are over an order of magnitude higher than any catalyst systems in the literature.3
| Entry | Aryl halide | Catalyst ([Pd]/mol% Pd) | Conversion (%)a | TON/103 (mol product/ mol Pd) |
|---|---|---|---|---|
| a Determined by GC, based on aryl halide. b 24 h reaction time. | ||||
| 1 | 4-Bromoacetophenone | 4a (10−4) | 90 | 900 |
| 2 | 4-Bromoacetophenone | 4a (10−5) | 20 | 2000 |
| 3 | 4-Bromoanisole | 4a (10−4) | 85 | 850 |
| 4 | 4-Bromoanisole | 4a (10−5) | 26 | 2600 |
| 5 | 4-Bromoanisole | 4b (10−4) | 91 | 910000 |
| 6 | 4-Bromoanisole | 4b (10−5) | 42 | 4200 |
| 7 | 4-Bromoanisole | 4c (10−4) | 29 | 290 |
| 8 | 4-Bromoanisole | 4a + 3a (10−4) | 100 | 1000 |
| 9 | 4-Bromoanisole | Pd(dba)2 + 2 3a (10−4) | 36 | 360 |
| 10 | 4-Bromoanisole | 4a + 3a (10−5) | 87.5 | 8750 |
| 11 | 4-Bromoanisole | 4b + 3b (10−5) | 50 | 5000 |
| 12 | 4-Bromoacetophenone | 4b + 3b (10−6)b | 100 | 100000 |
| 13 | 4-Bromoacetophenone | 4b + 3b (10−7)b | 47.5 | 475000 |
| 14 | 2-Bromotoluene | 4b + 3b (10−3) | 100 | 100 |
| 15 | 2-Bromo-m-xylene | 4b + 3b (10−3) | 48 | 48 |
| 16 | 4-Chloroanisole | 4b (1.0) | 6 | 0.006 |
Complex 4b generally shows higher activity than 4a, presumably due to the higher electron density that the phosphinite ligand confers on the palladium centre—this increase in electron density facilitates oxidative addition of the aryl bromide. The observation that 4a seems to show very little difference in activity in the coupling of phenylboronic acid with either 4-bromoacetophenone or 4-bromoanisole indicates that the oxidative addition of the aryl bromide may not be the rate determining step in the catalytic cycle. Comparing entries 3 and 7 it can be seen that decreasing the steric profile of the metallated ring has a deleterious effect on the rate of reaction, however 4c still shows substantially higher activity than its previously reported bis(phosphinite) PCP pincer counterpart, 2 (R = H).3 The importance of the metallation in the pre-catalysts is demonstrated by comparing entries 3, 8 and 9. The addition of a second phosphinite ligand to the metallated complex gives an increase in activity, by contrast the formation of bis(phosphinite) complexes in situ leads to substantially reduced reactivity. In other words addition of a second phosphinite is only beneficial if there is a metallated ring in the complex, otherwise it is deleterious. This demonstrates that the orthometallation of the pre-catalyst is extremely important; the possible role it plays is addressed below.
By contrast with the extraordinary results obtained with the deactivated aryl bromide 4-bromoanisole, complex 4b proved to be a very poor catalyst when 4-chloroanisole was used as a substrate, indeed substantial deposition of palladium metal was observed almost immediately upon heating. Interestingly, we found that rapid decomposition of all three complexes 4a–c occurs even if the 4-chloroanisole is left out of the reaction mixture. To gain evidence for the mechanism of decomposition a reaction was performed between 4c, PhB(OH)2 (4 molar equiv./Pd) and K2CO3 (5 molar equiv./Pd) in toluene at reflux temperature. The reaction was performed over 24 h, although decomposition started within seconds of the reaction reaching about 50 °C. After this time the supernatant liquid contained a mixture of compounds which included some unreacted 4c. A GC-MS spectrum of the mixture revealed the presence of some of the new phosphinite species 6c, this was confirmed by comparison with the spectrum obtained for a genuine sample of 6c. In addition, hydrolysis of the reaction mixture led to the formation of 2-phenylphenol, again demonstrated by GC-MS and compared with an authentic sample. A plausible mechanism of decomposition which results in the liberation of 6c and palladium metal is shown in Scheme 3.
 | ||
| Scheme 3 | ||
Since the complexes 4 show no reactivity with aryl bromides or chlorides in toluene at reflux temperature it is possible that the transmetallation and reductive elimination steps outlined in Scheme 3 also occur as initiation steps in the catalytic cycles of Suzuki reactions catalysed by these species and that the low coordinate mono-phosphinite species 7 represent the active catalysts in these reactions. Similar low coordinate species have been postulated as being the active catalysts in high activity Suzuki systems which employ palladium complexes of the phosphines 5.6 Part of the explanation given for the high activity of these latter complexes is that transient π-coordination of the secondary aryl ring of the ligand to the palladium centre may help to stabilise the low coordinate complex. The introduction of the oxygen atom into the ligands gives a higher degree of flexibility, thus it would be expected that the secondary aryl functions of the ligands 6a–c would participate in π-complexation with the metal centre even more readily than those in the ligands 5. Furthermore, due to higher π-acidity of the phosphorus donors, the ligands 6 would stabilise the zero-valent palladium complexes better than the ligands 5.
In summary, the catalysts described here show by far the highest activities yet reported in any Suzuki coupling reactions. These complexes are ideal catalysts for the coupling of deactivated and sterically hindered aryl bromides as they are comparatively inexpensive, very easily synthesised and can be used to give high conversions at extremely low concentrations. In addition preliminary results suggest that the active catalysts in these reactions may be low coordinate Pd(0) species.
Acknowledgements
We thank the EPSRC for funding and Johnson Matthey Chemicals for funding, the loan of palladium salts and access to their rapid catalyst screening programme.Notes and references
- M. Beller, H. Fischer, W. A. Herrmann, K. Öfele and C. Brossmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 1848 CrossRef CAS
.
- D. A. Albisson, R. B. Bedford, S. E. Lawrence and P. N. Scully, Chem. Commun., 1998, 2095 RSC
.
- R. B. Bedford, S. M. Draper, P. N. Scully and S. L. Welch, New J. Chem., 2000, 745 RSC
.
- H. Weissman and D. Milstein, Chem. Commun., 1999, 1901 RSC
.
- D. Zim, A. S. Gruber, G. Ebeling, J. Dupont and A. L. Monteiro, Org. Lett., 2000, 2, 2881 CrossRef CAS
.
- J. P. Wolfe, R. A. Singer, B. H. Yang and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 9550 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2001 |