Cellulose: a new bio-support for aqueous phase catalysts
Françoise Quignard and Agnès Choplin*
Institut de Recherches sur la Catalyse-CNRS, 2 avenue A. Einstein, 69 626, Villeurbanne Cedex, France.. E-mail: choplin@catalyse.univ-lyon1.fr
First published on 11th December 2000
Abstract
The choice of a hydrophilic bio-polymer, such as cellulose, as the support of the water soluble Pd(OAc)2/5 TPPTS system, leads to a new and efficient heterogeneous catalyst for the Trost Tsuji allylic alkylation reaction.
The heterogeneous catalysts designed for the synthesis of fine chemicals are usually supported either on inorganic oxides (silica, carbon..) or on organic synthetic polymers.1 If one excludes the case of supported metal particles modified with the appropriate asymmetric inductor (tartaric acid, cinchona alkaloids… ),2–5 most of these catalysts are anchored metal complexes; the anchoring bond links a surface atom either to the metal centre or to an atom of one of the ligands at a position as remote as possible from the active metal centre. The control of the selectivity of the surface anchoring reactions is one of the major difficulties; yet this affects directly the selectivity of the target organic reaction.
About ten years ago, the so-called Supported Aqueous Phase Catalysts were developed for the heterogenization of biphasic water/organic catalysts.6 This immobilisation procedure takes advantage of the hydrophilicity and of the high specific surface area of an inorganic support. The former character is necessary to maintain the catalyst-containing water film on the solid, the latter is responsible for the substantial activity increase as compared to that achieved in a biphasic medium. Silica is the most used support; mesoporous glass beads of controlled pore size distribution are the favourite, although the precise role of the porosity is so far not unambiguously demonstrated. This methodology is particularly well suited to the reactions non-feasible under conventional biphasic conditions such as, for example, those which involve water non-soluble reactants7 or water-sensitive reactions.8 We wish to report here the first example, to our knowledge, of a catalyst supported in an aqueous film on a natural polysaccharide, cellulose.
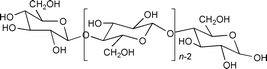
This polymer, which has unlimited availability as a renewable agro-resource and is biodegradable, presents a number of interesting structure related properties9 which have so far, to our knowledge, never been explored in the field of catalysis. Here, we have used its high hydrophilic character, induced by the presence of numerous hydroxy groups. Thus, we have immobilised on a cellulose powder the water soluble catalytic precursor Pd(OAc)2/5 TPPTS [TPPTS = P(m-C6H4SO3Na)3] using the SAP methodology and we have tested the catalytic properties of this solid for the allylic substitution of (E)- cinnamyl ethyl carbonate by morpholine [eqn. (1)].
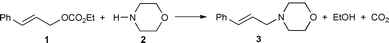 | (1) |
The cellulose powder (a generous gift from Institut Textile de France, Ecully, France, particles size: 80 μm) is characterised by a degree of crystallinity of 53% (determined by XRD), a specific surface area equal to 1.35 m2 g−1 (from the isotherm of N2 adsorption of the solid evacuated at 108 °C) and a wetting volume equal to 4.5 mL g−1 (defined as the volume of water which can be added to 1 g of solid before a drop of liquid can be visualised). All the manipulations are performed under inert atmosphere using classical Schlenk tube techniques. The catalyst is synthesised following the so-called incipient wetness method as described in ref. 10. Typically, Pd(OAc)2 (52.5 mg, 0.23 mmol) and TPPTS (732 mg, 1.3 mmol) are dissolved in de-aerated water (12 mL) under magnetic stirring (30 min, 40 °C). This solution is then poured onto cellulose (2.5 g, pre-evacuated at 120 °C (10 h, 10−4 Torr)) and the mixture stirred at 40 °C for 30 min. Water is then evacuated at rt (2 h at 10−1 Torr, 2 h at 10−4 Torr). The dry mustard yellow solid (CELL–SAP-Pd) is characterised by a 0.75% Pd content (determined by ICP) and a 3% wt water content (determined by TGA). The latter parameter is varied by addition of the desired quantity of water directly onto the solid with a syringe under magnetic stirring (rt, 30 min).
The catalytic tests are performed as described in ref. 10 under the following conditions: solvent: PhCN, T = 50 °C, [1]–[2]–[Pd] = 25∶30∶1; [1] = 30 mmol L−1. The reaction is followed by GPC (HP 5890, HP5 column, Tinj = 240 °C, Tdet. = 280 °C), calibrated against authentic samples. Analysis of the palladium content of the organic solutions at the end of each catalytic test is performed by ICP or ICP-MS when necessary.
The activity of CELL–SAP-Pd is strongly dependent on its water content (Fig. 1), a phenomenon which has already been observed with most silica–SAP catalysts.10–13 For these latter, one maximum is generally observed which seems to depend on the precise reaction (catalyst, reactants) under investigation. With CELL–SAP-Pd, two maxima of activity are evidenced. The first corresponds to a water content of 26 wt% or 0.5 g H2O/g CELL; 1 is converted after 100 min to a 60% level with a 100% selectivity for 3. The second is with 66 wt% H2O or 6 g H2O/g CELL; then the solid is much more active and 1 is fully converted to 3 within 100 min.
![Influence of the water amount (g H2O/g CELL) on the catalytic
activity of (Pd(OAc)2/5 TPPTS) supported on CELL, ◆:
tR = 50 min; ■: tR = 100 min
and on silica Δ (tR = 10 min. Exp. cond.:
solvent: PhCN, T = 50 °C, [1] = 30 mmol
L−1, [1]–[2]–[Pd] =
25∶30∶1.](/image/article/2001/CC/b007776n/b007776n-f1.gif) | ||
| Fig. 1 Influence of the water amount (g H2O/g CELL) on the catalytic activity of (Pd(OAc)2/5 TPPTS) supported on CELL, ◆: tR = 50 min; ■: tR = 100 min and on silica Δ (tR = 10 min. Exp. cond.: solvent: PhCN, T = 50 °C, [1] = 30 mmol L−1, [1]–[2]–[Pd] = 25∶30∶1. | ||
For the most active solid (66 wt% H2O), we tested the influence of the method of introduction of water on the solid. For this purpose, we added the desired water amount to the benzonitrile solution of the reactants. This biphasic solution is then poured on the ‘dry’ CELL–SAP-Pd sample. Instantaneously, the solution becomes monophasic but remains colourless; this confirms the high hydrophilicity of the surface of cellulose and suggests that palladium remains essentially on the solid. The activity of this sample is lower than that obtained with the general method (60 vs. 95% yield 3 after 100 min). But, we suspect that the main origin of this discrepancy is the uncontrolled agglomeration of the solid particles under the catalytic conditions; this necessarily induces major problems of diffusion of the reactants to the active centres. Accordingly, we observe also some irreproducibility of the activity of the solid CELL–SAP-Pd as soon as its water content lies above 1 g H2O/g CELL.
In none of these catalytic tests, is the presence of palladium detected in the organic catalytic solutions once the reactants are fully converted: the leaching of palladium, if any, is below 0.6%. This result is very encouraging because it is the first condition which must be met before one can envisage the possibility of recycling a supported catalyst, one of the advantages which is expected from the heterogenization of any molecular catalyst. It is then worth finding a way to improve the dispersion of the solid particles in the liquid organic medium.
In water, the surface of cellulose is anionic;9 therefore cationic surfactants should easily interact with the outer surface of the particles, and confer on them a hydrophobic character. This should facilitate their dispersion in an organic medium such as benzonitrile. When a small amount of cetyltrimethylammmonium bromide (CTAB) is added to the solution of the reactants, the dispersion of the solid particles CELL–Pd-SAP (2.6 g H2O/g CELL) is de visu excellent and concomitantly their activity increases sharply (Fig. 2). The increase of the concentration of CTAB (given as the initial concentration in PhCN) is beneficial for the activity, but concomitantly the palladium leaching increases up to an unacceptable level (Table 1). The addition of triethylpropylammonium bromide NEt3PrBr, which is not a surfactant but a common phase transfer agent in catalysis, improves neither the activity of the same solid, (Fig. 2) nor its dispersion, but simultaneously the palladium leaching is less severe (Table 1). Finally, we observe that CTAB, even at low concentrations, kills the activity of the related silica–SAP-Pd (40 wt% H2O) (Fig. 2).
![Influence of NEt3PrBr (◇) and of CTAB (◆) on
the activity of CELL–SAP-Pd (2.6 g H2O/g CELL and of CTAB
on the activity of silica–SAP-Pd (0.87 g H2O/g silica)
(Δ). tR = 5 min. Exp. cond.: solvent: PhCN,
T = 50 °C, [1]: 33 mmol L−1,
[1]–[2]–[Pd] = 25∶30∶1.](/image/article/2001/CC/b007776n/b007776n-f2.gif) | ||
| Fig. 2 Influence of NEt3PrBr (◇) and of CTAB (◆) on the activity of CELL–SAP-Pd (2.6 g H2O/g CELL and of CTAB on the activity of silica–SAP-Pd (0.87 g H2O/g silica) (Δ). tR = 5 min. Exp. cond.: solvent: PhCN, T = 50 °C, [1]: 33 mmol L−1, [1]–[2]–[Pd] = 25∶30∶1. | ||
| Ammonium bromide | |||
|---|---|---|---|
| Nature | Conc/mmol L−1 | Pd/ppm | % Pd |
| CTAB | 0 | 1.2 | 0.6 |
| 3 | 9 | 3 | |
| 9 | 14 | 8 | |
| 30 | 47 | 20 | |
| NEt3PrBr | 9 | 9 | 5 |
The interpretation of all these data clearly needs a more detailed investigation; yet, some important comments can already be made. The increase of the activity of CELL–SAP-Pd with its water content may be related to a greater mobility of the complex with increasing thickness of the water layer on the surface. But some properties must clearly be correlated to the nature of the support itself: water induces a swelling of the cellulose,9 a property which increases the surface accessibility. Thus for example, it is known that the specific surface of a cotton powder is very low when determined from the adsorption isotherm of N2, and raises values two orders of magnitude larger when determined from the adsorption of H2O.9 The double maximum may also reflect the structure of cellulose and more specifically the fact that the amorphous part is more accessible to the reactants than the crystalline part. Finally, CTAB clearly has a complex role, but it acts definitely as a surfactant, favouring the dispersion of the cellulose particles in the organic reaction medium and most probably also its swelling.
These data are very encouraging; they show the potential of cellulose as a support for the immobilisation of water soluble catalysts for fine chemical synthesis, these latter reactions are generally compatible with the thermal stability of this natural polymer. We are currently exploring the many other means of immobilisation of complexes which are possible on this type of solid, so as to take advantage of the specific chemical and structural properties of these polysaccharides as compared to the inorganic oxide supports traditionally used in the field of catalysis.
Acknowledgements
This work was financially supported by CNRS. M. R. Chatelin and P. Gayrine (ITF) are acknowledged for many encouraging and stimulating discussions.Notes and references
- For recent reviews, see: A. Choplin and F. Quignard, Coord. Chem. Rev., 1998, 178–180, 1679 and references therein. Search PubMed.
- Y. Orito, S. Imai and S. Niwa, J. Chem. Soc. Jpn., 1979, 8, 1118 Search PubMed.
- Y. Izumi, M. Iwaida, H. Fukawa and S. Akabori, Bull. Chem. Soc. Jpn., 1963, 36, 21.
- H.-U. Blaser, H. P. Jalett, M. Müller and M. Studer, Catal. Today, 1997, 37, 441 CrossRef CAS.
- T. Osawa, T. Harada and A. Tai, Catal. Today, 1997, 37, 465 CrossRef CAS.
- J. P. Arhancet, M. E. Davis, J. S. Merola and B. E. Hanson, Nature, 1989, 339, 454 CrossRef CAS.
- J. P. Arhancet, M. E. Davis and B. E. Hanson, J. Catal., 1991, 129, 100 CrossRef CAS.
- S. dos Santos, F. Quignard, D. Sinou and A. Choplin, Topics in Catalysis, 2000, 13, 311 Search PubMed.
- Comprehensive Cellulose Chemistry, vol. 1 Fundamentals and Analytical Methods, ed. D. Klemm, B. Philipp, T. Heinze, U. Heinze and W. Wagenknecht, Wiley-VCH, Weinheim, 1998. Search PubMed.
- S. dos Santos, Y. Tong, F. Quignard, A. Choplin, D. Sinou and J. P. Dutasta, Organometallics, 1998, 17, 78 CrossRef CAS.
- J. P. Arhancet, M. E. Davis, J. S. Merola and B. E. Hanson, J. Catal., 1990, 121, 327 CrossRef CAS.
- G. Frémy, E. Monflier, J. F. Carpentier, Y. Castanet and A. Mortreux, J. Mol. Catal., 1996, 162, 339 Search PubMed.
- B. B. Bunn, B. Barlik, W. R. Bebout, T. E. Glass and B. E. Hanson, J. Mol. Catal., 1994, 94, 157 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2001 |