Desolvated phosphate ions as acyl acceptors in dipolar aprotic media. A non-enzymatic model for formation of “energy-rich” acyl phosphates
Vanderlei Gageiro
Machado
a,
Clifford A.
Bunton
a,
César
Zucco
a and
Faruk
Nome
*a
aDepartamento de Química, Universidade Federal de Santa Catarina, Florianópolis, SC, 88040-900, Brazil. E-mail: E-mail: faruk@reitoria.ufsc.br
bDepartment of Chemistry, University of California, Santa Barbara, California 93106
First published on 13th January 2000
Abstract
n-Decyl phosphate, as the mono benzyltrimethylammonium salt 1, is readily acetylated by 2,4-dinitrophenyl acetate, 2, in dry acetonitrile (MeCN). Reaction is very strongly inhibited by H2O, and acyl phosphate, 3, is not detected with >20 vol% H2O. In these aqueous media ester 2 is hydrolyzed, probably with general-base catalysis by 1, and the acyl phosphate is also slowly hydrolyzed. Potassium dihydrogenphosphate is slowly acetylated by 2, in moist MeCN in the presence of [18]-crown-6 in heterogeneous conditions, but the yield of acetyl phosphate decreases sharply with >10 vol% H2O. Sodium n-decyl phosphate is also acetylated by 2 in dry MeCN in the presence of [15]-crown-5. These acetylations in anhydrous media model enzymic-mediated formation of acyl and other labile phosphates.
Introduction
Energy-rich phosphates (e.g., ATP and acetyl phosphate) have very important roles in energy transfers.1,2,3 The mechanisms and catalysis of hydrolysis of acetyl phosphate, and related energy-rich phosphate compounds, have been extensively studied,4 and Lehn et al.5 demonstrated that various macrocyclic polyamines mediate phosphorylation of phosphate ion.We recently reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 that desolvated n-decyl phosphate ion, 1, in anhydrous acetonitrile, reacts readily with 2,4-dinitrophenyl acetate, 2, to form the corresponding acyl phosphate 3 (Scheme 1), an “energy-rich” compound of biochemical interest. This reaction is strongly inhibited by addition of small amounts of water and, in aqueous solution, the products are acetate and 2,4-dinitrophenolate ions formed by hydrolysis of the ester. In this work, we report further mechanistic details of the reaction, with special emphasis on the role of water, acting as an inhibitor, and changing the course of reaction. These studies are aimed at providing mechanistic insight and chemical understanding of the role of water (or its absence) in biochemically important reactions. In Scheme 1 we also show formation of acetate and 2,4-dinitrophenolate ion by hydrolysis of the ester, 2, probably with general base catalysis by the phosphate ion, but we also have to consider possible hydrolysis of the acyl phosphate 3. We also attempted to acylate inorganic phosphate in relatively dry solvents, although here low ionic solubility is a major problem, and we used crown ethers in these experiments.
6 that desolvated n-decyl phosphate ion, 1, in anhydrous acetonitrile, reacts readily with 2,4-dinitrophenyl acetate, 2, to form the corresponding acyl phosphate 3 (Scheme 1), an “energy-rich” compound of biochemical interest. This reaction is strongly inhibited by addition of small amounts of water and, in aqueous solution, the products are acetate and 2,4-dinitrophenolate ions formed by hydrolysis of the ester. In this work, we report further mechanistic details of the reaction, with special emphasis on the role of water, acting as an inhibitor, and changing the course of reaction. These studies are aimed at providing mechanistic insight and chemical understanding of the role of water (or its absence) in biochemically important reactions. In Scheme 1 we also show formation of acetate and 2,4-dinitrophenolate ion by hydrolysis of the ester, 2, probably with general base catalysis by the phosphate ion, but we also have to consider possible hydrolysis of the acyl phosphate 3. We also attempted to acylate inorganic phosphate in relatively dry solvents, although here low ionic solubility is a major problem, and we used crown ethers in these experiments.
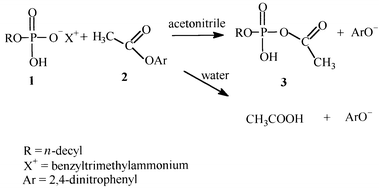 | ||
| Scheme 1 | ||
Experimental
Materials
2,4-Dinitrophenyl acetate, 2, and n-decylphosphoric acid were prepared according to reported methods.7,8Monopotassium phosphate (reagent grade, Reagen) and tris(hydroxymethyl)aminomethane (Tris; ultrapure grade, Aldrich) were used without further purification. Water was doubly distilled before use. Acetonitrile (HPLC grade, Aldrich) was dried with CaH2 (Aldrich), distilled and stored over molecular sieves (Aldrich 4 Å) as described.9 Karl Fischer titrations demonstrated the absence of water in the solvent.
Benzyltrimethylammonium n-decyl phosphate, 1, was prepared by addition of equimolecular benzyltrimethylammonium hydroxide (methanolic solution, 40 weight %; Aldrich) to aqueous n-decylphosphoric acid. The mixture was stirred for one hour, and solvent was removed in a rotary evaporator. The molten salt was dried under reduced pressure for 24 hours and stored over phosphorus pentoxide. An aqueous solution of 1, 5.5 × 10−3 mol dm−3, had pH 5.54.
Methods
Absorbance spectra (UV–visible) were measured with Perkin- Elmer Lambda 2S and Hewlett-Packard HP8452A spectrophotometers, equipped with thermostatted cell compartments to 0.1 °C. These instruments were also used for kinetic measurements. The pH was determined with a Hanna Model HI 9318 pH meter. Tris buffer (pH = 8.0) was used, except above 90% acetonitrile where it is insoluble.Kinetic runs were carried out under first-order conditions with at least a twenty-fold excess of 1. The stock ester solution was 0.01 mol dm−3 in anhydrous acetonitrile. Reactions were initiated by mixing 9 mm3 of this stock solution with thermally equilibrated 1 in a 3 cm3 quartz-stoppered cuvette (10 mm path length). The final concentration of 2 was 3 × 10−5 mol dm−3. Acetyl transfer was followed by the increase in absorbance at 363 nm (2,4-dinitrophenolate ion production). All reactions gave strict first-order kinetics over more than five half-lives. Observed first-order rate constants (kobs) were estimated from linear plots of ln (A − At) against time for at least 90% reaction by using an iterative least-squares program; correlation coefficients, r, were >0.999 for all kinetic runs. Unless specified otherwise, reactions were followed at 40 °C.
The activation parameters were determined from kobs values at six different temperatures, varying between 25 and 50 °C, by reacting 1 with 2, 1.57 × 10−3 mol dm−3, except the parameters calculated for anhydrous acetonitrile ([1] = 1.1 × 10−3 mol dm−3).
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12) and the solution was filtered. The solution became purple (500 nm) due to complexation of the hydroxamic acid with Fe3+ (FeCl3 does not absorb between 480 and 540 nm). We note that hydroxylamine reacts with 2 and it was necessary to follow formation of acetyl phosphate to completion by infrared or ultraviolet spectrometry before using this colorimetric method.
12) and the solution was filtered. The solution became purple (500 nm) due to complexation of the hydroxamic acid with Fe3+ (FeCl3 does not absorb between 480 and 540 nm). We note that hydroxylamine reacts with 2 and it was necessary to follow formation of acetyl phosphate to completion by infrared or ultraviolet spectrometry before using this colorimetric method.
Some experiments were made with KH2PO4 in MeCN or moist MeCN and, even in the presence of [18]-crown-6, reaction was in heterogeneous conditions. We examined the products by the method of Lipmann and Tuttle![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 12 and by IR spectrometry, as described above.
12 and by IR spectrometry, as described above.
Results and discussion
Reaction between 2,4-dinitrophenyl acetate and desolvated n-decyl phosphate ion
The reaction of desolvated n-decyl phosphate ion, 1, and 2,4-dinitrophenyl acetate, 2, occurs quantitatively at 40 °C (Fig. 1). Rate constants of acyl transfer were determined by following the appearance of 2,4-dinitrophenolate ion at 363 nm and the reaction is strictly second order with an isosbestic point at 316 nm. The first-order dependence on n-decyl phosphate concentration is limited only by the solubility of 1 in acetonitrile or acetonitrile–water. Plots of kobs against [n-decyl phosphate] in absence and presence of small amounts of water are linear with zero intercepts (Fig. 2), and give the second-order rate constants reported in Table 1 for 1 in the range 3.80–13.2 mol−1 s−1 dm3 (limited by the solubility of the salt). Second-order rate constants, k2, were reproducible to within ±2%. | ||
Fig. 1 The time course of reaction of 2,4-dinitrophenyl acetate (3.0 × 10−5 mol dm−3) and benzyltrimethylammonium n-decyl phosphate (1.1 × 10−3 mol dm−3) in anhydrous acetonitrile at 40 °C. Spectra were recorded at 10 (a), 30 (b), 59 (c), 120 (d), 150 (e), 270 (f![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) and 600 s (g) after mixing the reactants. The final spectrum is of the products. ) and 600 s (g) after mixing the reactants. The final spectrum is of the products.
| ||
 | ||
| Fig. 2 First-order rate constants for the reaction of 1 with 2 as a function of n-decyl phosphate concentration, at 40 °C, in acetonitrile with (▲) 0.06 M H2O; (•) 0.20 M H2O; (■) 0.44 M H2O. | ||
The absorbance at 363 nm at complete reaction was that of a solution of the equivalent amount of 2,4-dinitrophenolate and 1 in dry acetonitrile, demonstrating the stoichiometric formation of phenolate ion. In addition the IR signal of ester 2 at 1786 cm−1 disappears in the reaction with an excess of 1.
Formation of the acetyl phosphate, 3, is confirmed by examination of its infrared spectrum. Examination of the spectra as a function of time revealed that when the ester and n-decyl phosphate ion are mixed in acetonitrile, the carbonyl band of ester disappears, and a new band appears at 1754 cm−1. The appearance of the carbonyl signal, at 1754 cm−1, absent in the spectra of the individual starting materials, demonstrates the presence of the acetyl phosphate 3, since this signal is that of the carbonyl stretching vibration of acyl phosphates.10,11
Formation of acetyl phosphate was shown by its trapping, by an adaptation of the method of Lipmann and Tuttle.12 The molar absortivity of the complex of Fe3+–hydroxamic acid (εmax = 925 dm3 mol−1 cm−1, at 500 nm), was employed to demonstrate that the yield (>98 ±1%) of the acetyl phosphate is quantitative in anhydrous acetonitrile.
Kinetic effect of water on the reaction of 2 with n-decyl phosphate ion
Addition of small amounts of water inhibits the reaction between ester, 2, and n-decyl phosphate ion, 1. The kinetic data in Table 2 extend results previously reported![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 6 and confirm the strong inhibition of acyl transfer by water. These observations clearly demonstrate the key role of water in inhibiting acyl transfer, because solvation of phosphate anion by water strongly decreases its nucleophilicity.13 The preferential solvation of ions by water in mixed solvents is well documented in the literature, and many examples illustrate the importance of specific solvation of cations and desolvation of anions in dipolar aprotic solvents.10,11,14
6 and confirm the strong inhibition of acyl transfer by water. These observations clearly demonstrate the key role of water in inhibiting acyl transfer, because solvation of phosphate anion by water strongly decreases its nucleophilicity.13 The preferential solvation of ions by water in mixed solvents is well documented in the literature, and many examples illustrate the importance of specific solvation of cations and desolvation of anions in dipolar aprotic solvents.10,11,14
| [H2O]/mol dm−3 | 103kobs/s−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
|---|---|
| a kobs values are within 2% of the mean. b Value extrapolated from the second-order constant for reaction in anhydrous acetonitrile (Table 1). c In buffer Tris 5.0 × 10−2 mol dm−3; pH = 8.0. d In the absence of buffer. | |
| 0.00 | 15.0![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
| 0.06 | 10.5 |
| 0.11 | 9.55 |
| 0.20 | 7.74 |
| 0.33 | 7.01 |
| 0.44 | 5.80 |
| 0.66 | 3.81 |
| 0.88 | 2.70 |
| 1.10 | 2.14 |
| 1.28 | 1.57 |
| 1.76 | 1.03 |
| 2.21 | 0.393 |
| 2.65 | 0.221 |
| 3.55 | 0.177 |
4.46![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
0.0548 |
4.46![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
0.0227 |
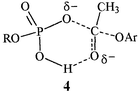
The reaction involves an anionic transition state, 4, which may be similar in structure to a hypothetical tetrahedral intermediate and in that event negative charge should be stabilized by interaction with the hydrogenphosphate.
Activation parameters
Activation parameters were calculated for the reaction of 2,4-dinitrophenyl acetate with n-decyl phosphate in acetonitrile in the absence and presence of defined amounts of water (Fig. 3). Values of ΔG![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡, ΔH
‡, ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ and ΔS
‡ and ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ are calculated at 40 °C (Table 3). Values of ΔH
‡ are calculated at 40 °C (Table 3). Values of ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ increase with increasing concentration of water in the reaction medium, reflecting the sharp decrease in nucleophilicity of n-decyl phosphate ion. Changes in ΔS
‡ increase with increasing concentration of water in the reaction medium, reflecting the sharp decrease in nucleophilicity of n-decyl phosphate ion. Changes in ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ are significant: values are high and negative (−169.0 and −210.9 J K−1 mol−1, in 99 and 100% acetonitrile, respectively). Indeed, addition of 1 vol% water makes ΔS
‡ are significant: values are high and negative (−169.0 and −210.9 J K−1 mol−1, in 99 and 100% acetonitrile, respectively). Indeed, addition of 1 vol% water makes ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ less negative by ca. 42 J K−1 mol−1, probably because of release of water in forming transition state 4. The negative ΔS‡ suggests a highly associative mechanism by which the ester interacts with the n-decyl phosphate ion. A good relationship is obtained between ΔH
‡ less negative by ca. 42 J K−1 mol−1, probably because of release of water in forming transition state 4. The negative ΔS‡ suggests a highly associative mechanism by which the ester interacts with the n-decyl phosphate ion. A good relationship is obtained between ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ and ΔS‡ (Table 3, plot not included); the data show that a decrease in TΔS
‡ and ΔS‡ (Table 3, plot not included); the data show that a decrease in TΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ is thermally balanced by a decrease in ΔH
‡ is thermally balanced by a decrease in ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡. Thus, the enthalpy changes are almost completely lost in ΔG
‡. Thus, the enthalpy changes are almost completely lost in ΔG![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡. We believe that the experimental result comes from a balance of the increase in nucleophilicity of the phosphate ion and the relative inability of water to solvate the charge-dispersed transition state, 4.
‡. We believe that the experimental result comes from a balance of the increase in nucleophilicity of the phosphate ion and the relative inability of water to solvate the charge-dispersed transition state, 4.
| [H2O]/mol dm−3 |
E
a/kJ mol−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡/kJ mol−1 ‡/kJ mol−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
ΔS‡/J K−1 mol−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
ΔG![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡/kJ mol−1 ‡/kJ mol−1 |
|---|---|---|---|---|
a
Ea and ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ values are accurate to within ±1 kJ mol−1.
b
ΔS‡ values are accurate to within ±3.3 J K−1 mol−1. ‡ values are accurate to within ±1 kJ mol−1.
b
ΔS‡ values are accurate to within ±3.3 J K−1 mol−1.
|
||||
| 0.44 | 39.8 | 37.1 | −169 | 73.3 |
| 0.33 | 36.5 | 33.9 | −178 | 72.8 |
| 0.20 | 34.2 | 31.6 | −184 | 72.4 |
| 0.11 | 29.4 | 26.8 | −198 | 71.9 |
| 0.06 | 27.7 | 25.1 | −203 | 71.6 |
| 0.00 | 25.6 | 23.0 | −211 | 70.1 |
 | ||
| Fig. 3 Influence of temperature on kobs for the reaction between 2,4-dinitrophenyl acetate and benzyltrimethylammonium n-decyl phosphate in acetonitrile with 0.13 (▽), 0.25 (✦), 0.46 (•), and 1% (■) of water. | ||
There is no net change of charge in formation of the transition state, but there is extensive delocalization, with a possible interaction of a phosphate hydrogen with the carbonyl oxygen. Formation of such a transition state will be entropically costly, but the partial charge neutralization will decrease ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡.
‡.
A comparison between our data and the activation data reported in the literature for the pyridine-catalyzed hydrolysis of ester 2 (Table 4) shows a good agreement, with high negative values of the entropy of activation and low values of the activation enthalpy, in both cases.
| Solvent | Conditions | ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ kJ mol−1 ‡ kJ mol−1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
ΔS‡/e.u.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
|---|---|---|---|
a
ΔH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ values are accurate to within ±1 kJ mol−1.
b
ΔS ‡ values are accurate to within ±1 kJ mol−1.
b
ΔS![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ‡ values are accurate to within to ±0.5 eu.
c
A. R. Butler and I. H. Robertson, J. Chem. Soc., Perkin Trans. 2, 1975, 660.
d
W. P. Jencks and M. Gilchrist, J. Am. Chem. Soc., 1968, 90, 2622.
e
I. M. Kovach, J. Org. Chem., 1982, 47, 2235. ‡ values are accurate to within to ±0.5 eu.
c
A. R. Butler and I. H. Robertson, J. Chem. Soc., Perkin Trans. 2, 1975, 660.
d
W. P. Jencks and M. Gilchrist, J. Am. Chem. Soc., 1968, 90, 2622.
e
I. M. Kovach, J. Org. Chem., 1982, 47, 2235.
|
|||
Water![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
Pyridine-catalyzed | 36.4 | −30.0 |
Water![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
Alkaline solution | 46.0 | −13.4 |
Water![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
With morpholine | 38.1 | −19.0 |
MeCN![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
Crown-ether/KAc | 43.5 | 9.3 |
Benzene![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
Crown-ether/KAc | 23.0 | 19.4 |
| MeCN | Our results | 23.0 | −36.0 |
| MeCN–water 1% | Our results | 37.2 | −27.6 |
Influence of water on overall reactions between 2,4-dinitrophenyl acetate and n-decyl phosphate ion
Reaction of 2 with 1 in anhydrous acetonitrile quantitatively forms the acetyl phosphate 3 (Table 5). In buffered aqueous solution (pH = 8.0) with KH2PO4, 0.1 mol dm−3, the reaction is exclusively ester hydrolysis. The strong influence of water on the course of these reactions between the ester and n-decyl phosphate, is shown by the yields of products (Table 5). Addition of 5 vol% of water to acetonitrile markedly reduces the yield of the acetyl phosphate. We could not detect it in 30 vol% water by Lipmann and Tuttle’s test, or by infrared spectral analysis, and finding only hydrolysis products of the ester.![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a of acetyl phosphate and hydrolysed ester in the reaction between 1 and 2 in acetonitrile–water, at 25 °C
a of acetyl phosphate and hydrolysed ester in the reaction between 1 and 2 in acetonitrile–water, at 25 °C
| Water(%) | Acetyl phosphate yield(%) | Hydrolysed ester yield(%) |
|---|---|---|
| a The yields are accurate to within ±2%. | ||
| 0.00 | 100 | 0 |
| 0.25 | 95 | 5 |
| 0.46 | 90 | 10 |
| 1.00 | 86 | 14 |
| 1.50 | 76 | 24 |
| 3.00 | 71 | 29 |
| 4.00 | 63 | 37 |
| 5.00 | 52 | 48 |
| 6.00 | 44 | 56 |
| 10.00 | 37 | 63 |
| 15.00 | 24 | 76 |
| 20.00 | 15 | 85 |
| 30.00 | 0 | 100 |
| 40.00 | 0 | 100 |
| 50.00 | 0 | 100 |
The yields reported in Table 5 may not represent all the acetyl phosphate, 3, formed during reaction, because some of it may hydrolyze. A test of the stability of 3 in acetonitrile with various water contents, showed that with 5, 10 and 20 vol% water, 3 suffered about 10, 20 and 40% hydrolysis over 72 hours, at 25 °C. As the concentration of water increases, the kinetic stability of 3 decreases and its hydrolysis products are detected. However, in the range of 0–10 vol% water, the results in Table 5 reflect only formation of 3 in the reaction of ester, 2, with 1, with essentially no hydrolysis. The failure to detect acyl phosphate, 3, in aqueous media may be due to its rapid hydrolysis.
KH2PO4 is too insoluble in anhydrous acetonitrile, even in the presence of crown ethers, to allow a study similar to that reported for 2 (Scheme 1). Nonetheless, after stirring a solution of 2 in acetonitrile, in the presence of [18]-crown-6, with added KH2PO4, for 72 hours, we saw a weak IR signal at 1754 cm−1, which was not present in these conditions without crown ether. These results indicate that the crown ether solubilizes some inorganic phosphate, which reacts in solution with the ester. This reaction, however, is very slow and we could not estimate the yield.
We then carried out a similar experiment, but with addition of a little water. The salt did not appear to dissolve, but reaction was faster. Addition of 2 and 5 vol% of water gave acetyl phosphate in 60 and 50% yield, respectively. The product was confirmed by its infrared spectrum and quantified by the Lipmann and Tuttle method. The yield of acetyl phosphate decreases strongly with an increase in water content, and is 40% with 10 vol% water. Acetyl phosphate was not detected by the method of Lipmann and Tuttle in 30 vol% water, but there was a weak signal of the carbonyl group at 1754 cm−1.
We also carried out the same experiment with sodium n-decyl phosphate in the presence of [15]-crown-5, in acetonitrile–Tris buffer (pH 8.0). The acyl phosphate was obtained in 45% yield, confirmed by its infrared spectrum and quantified by the method of Lipmann and Tuttle. These results show that the benzyltrimethylammonium ion is not essential in the acyl transfer. Its major role is to solubilize the phosphate ion, but it may assist reaction by interacting with the low charge density transition state 4.
On the role of water in the formation of acetyl phosphates
The non-enzymatic model described here represents the first bioorganic system that mimics the synthesis of energy-rich acyl phosphates. The strong dependence of the formation of the acyl phosphate, 3, on the molar composition of the medium demonstrates the importance of the solvent, not only in enhancing the nucleophilicity of the organic phosphate, but also in changing the course of the reaction.For many reasons, the study of reactivity of compounds in a dipolar aprotic media provides an important insight into the understanding of a model system, in comparison to water alone as solvent.15 Water has both basic and acidic properties, which are responsible for changes in the relative free energies of the transition and ground states. These interactions of water are difficult to detect if it is present in large excess as the bulk solvent. However, at low concentrations in acetonitrile, the tri-dimensional hydrogen bonded structure of water is broken and mobile acetonitrile–water clusters predominate in solution, with the water molecules hydrogen-bonded to acetonitrile,16,17 but isolated water molecules will solvate a nucleophile because they are stronger proton donors than acetonitrile. The striking decrease in the nucleophilic reactivities of the phosphate ions in acetonitrile by added water is largely due to anionic stabilization by hydrogen bonding. Water strongly inhibits acylation, showing that this kind of interaction is much weaker in the transition state and the dominant effect of ground-state stabilization is well documented for reactions of anionic nucleophiles and bases.14c,d
Although acyl phosphates are regarded as “energy-rich” they can be prepared from inorganic phosphate, or an alkyl phosphate ion, by the weak acylating agent 2,4-dinitrophenyl acetate, 2, provided that the reactions are not prohibited by hydration of the phosphate ions. Aqueous media change the course of reaction, since solvation promotes simultaneously a decrease in nucleophilicity, via hydrogen bonding, and favors phosphate ions action as general bases, activating water towards the ester, 2. Insofar as the active site of an enzyme can have a very low water content, the driving force for acylation of a phosphate ion, or similar reactions, can be related to anionic desolvation.
The descriptively useful term “energy-rich” is applied to labile phosphates, e.g., acyl phosphates and triphosphates, which transfer the phosphoryl moiety in biologically important reactions. It is important to remark that although the model chemistry in acetonitrile is not exact, since in the enzyme phosphate would be bound to favorably disposed enzyme groups, the experimental results demonstrate that solvation plays a major role in this type of reaction. Emphasis is often given to free energy changes in equilibrium conditions, but the enzymic (and model) reactions are typically not carried out in these conditions, although they involve an overall decrease in free energy.18 We believe that it is desirable to analyze these reactions in terms of the kinetics of the individual steps, and in particular to consider the extent to which the presence, or absence, of water is of major kinetic importance and can change the course of reaction.
Acknowledgements
The authors are grateful to PRONEX, CNPq and PADCT/FINEP in Brazil and the National Science Foundation (International Programs) for financial support of this work.References
- F. Lipmann, Adv. Enzymol., 1941, 1, 99 Search PubMed.
- (a) L. de Meis, Biochim. Biophys. Acta, 1989, 973, 333 CAS; (b) L. de Meis, Arch. Biochem. Biophys., 1993, 306, 287 CrossRef CAS.
- (a) H. S. Penefsky and R. L. Cross, Adv. Enzymol. Relat. Areas Mol. Biol., 1991, 64, 173 Search PubMed; (b) P. D. Boyer, Biochim. Biophys. Acta, 1993, 1140, 215 CAS; (c) R. L. Cross, Nature, 1994, 370, 594 CrossRef CAS; (d) J. P. Abrahams, A. G. W. Leslie, R. Lutter and J. E. Walker, Nature, 1994, 370, 621 CrossRef CAS.
- (a) D. E. Koshland, Jr., J. Am. Chem. Soc., 1952, 74, 2286 CrossRef; (b) G. di Sabato and W. P. Jencks, J. Am. Chem. Soc., 1961, 83, 4400 CrossRef CAS; (c) A. J. Kirby and A. G. Varvoglis, J. Am. Chem. Soc., 1966, 89, 415; (d) D. R. Phillips and T. H. Fife, J. Am. Chem. Soc., 1968, 90, 6803 CrossRef CAS; (e) F. Ramirez and J. F. Marecek, Pure Appl. Chem., 1980, 52, 1021 CrossRef CAS; (f) D. Hertschlag and W. P. Jencks, J. Am. Chem. Soc., 1986, 108, 7938 CrossRef; (g) H. Sigel, Chimia, 1987, 41, 11 Search PubMed; (h) G. P. Haight, Jr., Coord. Chem. Rev., 1987, 79, 293 CrossRef; (i) L. de Meis and V. A. Suzano, FEBS Lett., 1988, 232, 73 CrossRef CAS; (j) H. Sigel and R. J. Tribolet, Inorg. Biochem., 1990, 40, 163 Search PubMed.
- (a) J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89 CrossRef; (b) M. W. Hosseini and J.-M. Lehn, J. Chem. Soc., Chem. Commun., 1991, 451 RSC; (c) H. Fenniri and J.-M. Lehn, J. Chem. Soc., Chem. Commun., 1993, 1819 RSC.
- V. G. Machado and F. Nome, Chem. Commun., 1997, 1917 RSC.
- F. Chattaway, J. Chem. Soc., 1931, 134, 2495 RSC.
- G. Imokawa and H. Tsutsumi, J. Am. Oil Chem. Soc., 1979, 55, 839 Search PubMed.
- S. Furniss , A. J. Hannaford , P. W. G. Smith and A. R. Tatchell , Vogel’s Textbook of Practical Organic Chemistry, Longman, London, 1989, 5th edn., p. 410. Search PubMed.
- J. Hajdu and G. M. Smith, J. Am. Chem. Soc., 1980, 102, 3960 CrossRef CAS; J. Hajdu and G. M. Smith, J. Am. Chem. Soc., 1981, 103, 6192 CrossRef CAS.
- G. Wallerberg and P. Haake, J. Org. Chem., 1981, 46, 43 CrossRef CAS.
- F. Lipmann and L. C. Tuttle, J. Biol. Chem., 1945, 159, 21 CAS.
- (a) W. P. Jencks, Chem. Rev., 1972, 72, 705 CrossRef CAS F. M. Menger , in Nucleophilicity, ed. J. M. Harris and S. P. McManus, American Chemical Society, Washington, 1987, p. 209; Search PubMed (b) M. J. S. Dewar and D. M. Storch, J. Chem. Soc., Chem. Commun., 1985, 94 RSC; (c) J. Chandrasekhar, S. F. Smith and W. L. Jorgensen, J. Am. Chem. Soc., 1985, 107, 154 CrossRef CAS.
- (a) I. M. Kolthoff and M. K. Chantooni, J. Am. Chem. Soc., 1976, 98, 7465 CrossRef CAS; (b) I. M. Kovach, Tetrahedron Lett., 1980, 21, 4309 CrossRef CAS; (c) E. Buncel, E. J. Dunn, N. van Truong, R. A. B. Bannard and J. G. Purdon, Tetrahedron Lett., 1990, 31, 6513 CrossRef CAS; (d) E. Buncel, A. Kumar, H.-Q. Xie, R. Y. Moir and J. G. Purdon, Can. J. Chem., 1994, 72, 437 CAS; (e) M. Scremin, S. P. Zanotto, V. G. Machado and M. C. Rezende, J. Chem. Soc., Faraday Trans., 1994, 90, 865 RSC.
- C. D. Ritchie , in Solute-Solvent Interactions, ed. J. F. Coetzee and C. D. Ritchie, Marcel Dekker, New York, 1969, p. 219. Search PubMed.
- J. F. Coetzee, Prog. Phys. Org. Chem., 1967, 4, 45 Search PubMed.
- Y. Marcus and Y. Migron, J. Phys. Chem., 1991, 95, 400 CrossRef CAS.
- A. J. Kirby, Angew. Chem., Int. Ed. Engl., 1996, 35, 707 CrossRef CAS; A. J. Kirby, Acc. Chem. Res., 1997, 30, 290 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |