Novel xanthine oxidase inhibitor studies. Part 3.1 Convenient and general syntheses of 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) )-ones as a new class of potential xanthine oxidase inhibitors
)-ones as a new class of potential xanthine oxidase inhibitors
Tomohisa
Nagamatsu
*a,
Takayuki
Fujita
a and
Kazuki
Endo
b
aFaculty of Pharmaceutical Sciences, Okayama University, Tsushima, Okayama, 700-8530, Japan
bBiology Laboratory, Research and Development Division, Yamasa Syoyu Co., Choshi, Chiba, 288-0056, Japan
First published on 12th January 2000
Abstract
Convenient and general syntheses of 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (12), a new class of potent xanthine oxidase inhibitors, involving the oxidative cyclisation of 6-substituted 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d
)-ones (12), a new class of potent xanthine oxidase inhibitors, involving the oxidative cyclisation of 6-substituted 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidines (3 and 11) with 70% nitric acid as the key step, are reported. The hydrazones 3 and 11 were obtained by a versatile synthetic route via the key intermediates, 6-chloro-4-hydrazino-1H-pyrazolo[3,4-d
]pyrimidines (3 and 11) with 70% nitric acid as the key step, are reported. The hydrazones 3 and 11 were obtained by a versatile synthetic route via the key intermediates, 6-chloro-4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidine 2 or oxypurinol 4, starting from 2,4,6-trichloropyrimidine-5-carbaldehyde 1. Their inhibitory activities against bovine milk xanthine oxidase in vitro are also described; i.e. the pyrazolotriazolopyrimidines 12 were several hundred times more potent than allopurinol.
]pyrimidine 2 or oxypurinol 4, starting from 2,4,6-trichloropyrimidine-5-carbaldehyde 1. Their inhibitory activities against bovine milk xanthine oxidase in vitro are also described; i.e. the pyrazolotriazolopyrimidines 12 were several hundred times more potent than allopurinol.
Introduction
Allopurinol, a well known drug clinically used for treatment of gout and hyperuricemia resulting from uric acid,2–4 has been reported as a potential inhibitor of xanthine oxidase (XO), which catalyzes the conversion of hypoxanthine and xanthine to uric acid.5 Allopurinol is relatively non-toxic and does not appear to interfere with anabolic processes within the cell, as judged by its lack of inhibition of the growth of bacteria or tumors.6 However, some allopurinol toxicities![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 7 and a life-threatening toxicity syndrome have been reported after its use.8 Although XO inhibitory activities have recently been discovered in some newly synthesized compounds and previously known compounds,9–15 no clinically effective XO inhibitors for the treatment of hyperuricemia have been developed since allopurinol was introduced for clinical use in 1963.2,6 We have recently discovered that 6-alkylidenehydrazino- or 6-arylmethylidenehydrazino-7H-purines (I) and the angular type purine analogues, 9H-1,2,4-triazolo[3,4-i]purines (II), have exhibited more potent bovine milk XO inhibitory activities than that of allopurinol.1,16,17
7 and a life-threatening toxicity syndrome have been reported after its use.8 Although XO inhibitory activities have recently been discovered in some newly synthesized compounds and previously known compounds,9–15 no clinically effective XO inhibitors for the treatment of hyperuricemia have been developed since allopurinol was introduced for clinical use in 1963.2,6 We have recently discovered that 6-alkylidenehydrazino- or 6-arylmethylidenehydrazino-7H-purines (I) and the angular type purine analogues, 9H-1,2,4-triazolo[3,4-i]purines (II), have exhibited more potent bovine milk XO inhibitory activities than that of allopurinol.1,16,17
In our recent communication,18 we reported the facile and general syntheses of 3- and/or 5-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidines (III) as a new class of potential xanthine oxidase inhibitors. Herein we report full details of the versatile and general syntheses of the 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (III), involving the oxidative cyclisation of 6-substituted 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d
)-ones (III), involving the oxidative cyclisation of 6-substituted 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidines as the key step. Furthermore, we also report here their inhibitory activities against bovine milk xanthine oxidase in comparison with allopurinol in vitro.
]pyrimidines as the key step. Furthermore, we also report here their inhibitory activities against bovine milk xanthine oxidase in comparison with allopurinol in vitro.
Results and discussion
In the preceding paper,1 we have clarified that 9H-1,2,4-triazolo[3,4-i![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]purines (II), especially the 5-oxo or 5-thioxo derivatives, showed more potent bovine milk XO inhibitory activities than allopurinol. Therefore, in this paper we tried to prepare 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H
]purines (II), especially the 5-oxo or 5-thioxo derivatives, showed more potent bovine milk XO inhibitory activities than allopurinol. Therefore, in this paper we tried to prepare 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (III), which are analogous to the triazolopurines (II), as another new class of potential XO inhibitors. Few methods for synthesis of the pyrazolotriazolopyrimidines (III) have been reported in the journal
)-ones (III), which are analogous to the triazolopurines (II), as another new class of potential XO inhibitors. Few methods for synthesis of the pyrazolotriazolopyrimidines (III) have been reported in the journal![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 19,20 or patent
19,20 or patent![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 21 literature and several derivatives have been synthesised. However, none of the 5-substituted derivatives has been prepared up to now.
21 literature and several derivatives have been synthesised. However, none of the 5-substituted derivatives has been prepared up to now.
In the first place we tried to synthesise the key intermediate, 4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
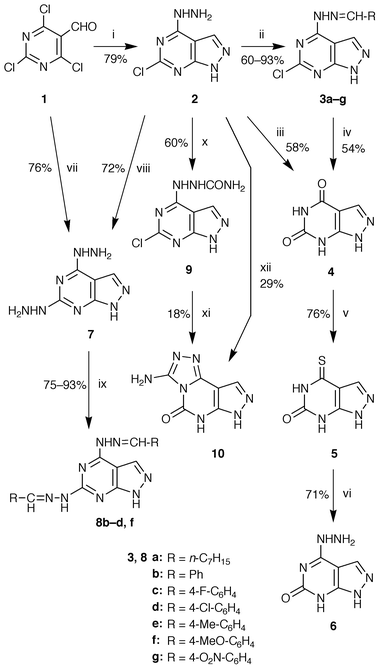
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 6 derived from barbituric acid. The requisite starting material, 2,4,6-trichloropyrimidine-5-carbaldehyde 1, was prepared according to a literature method.22 Treatment of 1 with anhydrous hydrazine (4 equiv.) in 2-methoxyethanol at 0 °C afforded 6-chloro-4-hydrazino-1H-pyrazolo[3,4-d
)-one 6 derived from barbituric acid. The requisite starting material, 2,4,6-trichloropyrimidine-5-carbaldehyde 1, was prepared according to a literature method.22 Treatment of 1 with anhydrous hydrazine (4 equiv.) in 2-methoxyethanol at 0 °C afforded 6-chloro-4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidine 2 in 79% yield (Scheme 1). Subsequent reaction of compound 2 with appropriate aldehydes (1.2 equiv.) in dimethylfumamide (DMF) at room temperature gave the corresponding hydrazones 3a–g in 60–93% yields as shown in Tables 1 and 2. Further, heating compound 2 in concentrated hydrochloric acid (50 parts) under reflux for 1 hour gave oxypurinol 4 (58% yield), which was confirmed by direct comparison with an authentic sample.23 The oxypurinol 4 was also obtained in a similar yield by heating the hydrazone 3b (R = Ph) in 10% hydrochloric acid (100 parts) for 3 hours. Thiation of oxypurinol 4 by phosphorous pentasulfide gave the 6-oxo-4-thioxo derivative 5 (76% yield) following a literature procedure
]pyrimidine 2 in 79% yield (Scheme 1). Subsequent reaction of compound 2 with appropriate aldehydes (1.2 equiv.) in dimethylfumamide (DMF) at room temperature gave the corresponding hydrazones 3a–g in 60–93% yields as shown in Tables 1 and 2. Further, heating compound 2 in concentrated hydrochloric acid (50 parts) under reflux for 1 hour gave oxypurinol 4 (58% yield), which was confirmed by direct comparison with an authentic sample.23 The oxypurinol 4 was also obtained in a similar yield by heating the hydrazone 3b (R = Ph) in 10% hydrochloric acid (100 parts) for 3 hours. Thiation of oxypurinol 4 by phosphorous pentasulfide gave the 6-oxo-4-thioxo derivative 5 (76% yield) following a literature procedure![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 23 and the reaction of 5 with excess 50% ethanolic hydrazine under reflux yielded the desired intermediate, 4-hydrazino-1H-pyrazolo[3,4-d
23 and the reaction of 5 with excess 50% ethanolic hydrazine under reflux yielded the desired intermediate, 4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 6, in 71% yield.
)-one 6, in 71% yield.
| (Found (%) (Required) | |||||||
|---|---|---|---|---|---|---|---|
| Compound (Formula) | Yield (%) | Mp/°C | Recrystn. solvent![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a (Rf, solvent system a (Rf, solvent system![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b) b) |
C | H | N | m/z MH + |
| a All compounds 3 and 8 were obtained as colourless or pale yellow powdery crystals except for 3g (yellow). b Solvent systems: (A) AcOEt–n-hexane (4∶3 v/v), (B) AcOEt–EtOH (9∶1 v/v). | |||||||
| 3a | 60 | 250 | AcOEt | 52.9 | 6.5 | 28.5 | 295/297 |
| C13H19ClN6 | (0.78, A) | (53.0) | (6.5) | (28.5) | |||
| 3b | 93 | >300 | DMF | 52.4 | 3.7 | 30.6 | 273/275 |
| C12H9ClN6 | (0.56, A) | (52.85) | (3.3) | (30.8) | |||
| 3c | 89 | >300 | EtOH–DMF | 49.9 | 2.8 | 29.2 | 291/293 |
| C12H8ClFN6 | (0.60, A) | (49.6) | (2.8) | (28.9) | |||
| 3d | 76 | >300 | EtOH–DMF | 46.7 | 2.4 | 27.0 | 307/309/311 |
| C12H8Cl2N6 | (0.64, A) | (46.9) | (2.6) | (27.4) | |||
| 3e | 78 | >300 | EtOH–DMF | 54.0 | 4.1 | 29.1 | 287/289 |
| C13H11ClN6 | (0.61, A) | (54.5) | (3.9) | (29.3) | |||
| 3f | 79 | >300 | EtOH–DMF | 51.7 | 3.8 | 27.7 | 303/305 |
| C13H11ClN6O | (0.57, A) | (51.6) | (3.7) | (27.8) | |||
| 3g | 70 | >300 | EtOH–DMF | 45.6 | 2.7 | 30.6 | 318/320 |
| C12H8ClN7O2 | (0.49, A) | (45.4) | (2.5) | (30.9) | |||
| 8b | 87 | 298–300 | EtOH–DMF | 60.7 | 4.8 | 29.9 | 357 |
| C19H16N8·H2O | (0.60, B) | (60.95) | (4.85) | (29.9) | |||
| 8c | 93 | >300 | EtOH–DMF | 54.1 | 4.1 | 26.4 | 393 |
| C19H14F2N8·3/2 H2O | (0.61, B) | (54.4) | (4.1) | (26.7) | |||
| 8d | 75 | >300 | EtOH–DMF | 50.2 | 3.7 | 24.6 | 425/427/429 |
| C19H14Cl2N8·3/2 H2O | (0.68, B) | (50.45) | (3.8) | (24.8) | |||
| 8f | 77 | 276 | EtOH–DMF | 57.7 | 5.1 | 25.7 | 417 |
| C21H20N8O2·H2O | (0.62, B) | (58.1) | (5.1) | (25.8) | |||
| Compound | ν max(Nujol)/cm−1 | δ H[60 MHz; (CD3)2SO; Me4Si] |
|---|---|---|
| a This compound was measured at 200 MHz. | ||
| 3a | 3180, 3100 (NH) | 0.86 (3 H, J 6.8, CHCH2[CH2]5CH3), 1.31 (10 H, br s, CHCH2[CH2]5CH3), 2.15–2.60 (2 H, m, CHCH2[CH2]5CH3), 7.61 (1 H, t, J 6.6, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) CH2[CH2]5CH3), 8.18 (1 H, s, 3-H), 12.00 (1 H, br, 4-NH), 12.90 (1 H, br, 1-NH) CH2[CH2]5CH3), 8.18 (1 H, s, 3-H), 12.00 (1 H, br, 4-NH), 12.90 (1 H, br, 1-NH) |
| 3b | 3190, 3080 (NH) | 7.40–7.60 (3 H, m, Ph-m,pH), 7.70–7.95 (2 H, m, Ph-oH), 8.28 (1 H, s, 3-H), 8.40 (1 H, s, CH-Ar), 12.44 (1 H, s, 4-NH), 13.75 (1 H, br s, 1-NH) |
| 3c | 3200, 3100 (NH) | 7.32 (2 H, dd, JH,H 8.8, JH,F 9.1, Ar-mH), 7.90 (2 H, dd, JH,H 8.8, JH,F 5.9, Ar-oH), 8.27 (1 H, s, 3-H), 8.40 (1 H, s, CH-Ar), 12.45 (1 H, s, 4-NH), 13.70 (1 H, br, 1-NH) |
3d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3180, 3080 (NH) | 7.55 (2 H, d, J 8.6, Ar-mH), 7.85 (2 H, d, J 8.6, Ar-oH), 8.26 (1 H, s, 3-H), 8.40 (1 H, s, CH-Ar), 12.54 (1 H, br, 4-NH), 13.70 (1 H, br, 1-NH) |
| 3e | 3190, 3080 (NH) | 2.37 (3 H, s, CH3), 7.30 (2 H, d, J 7.9, Ar-mH), 7.70 (2 H, d, J 7.9, Ar-oH), 8.29 (1 H, s, 3-H), 8.38 (1 H, s, CH-Ar), 12.40 (1 H, br, 4-NH), 13.50 (1 H, br, 1-NH) |
| 3f | 3210, 3100 (NH) | 3.84 (3 H, s, OCH3), 7.06 (2 H, d, J 8.8, Ar-mH), 7.78 (2 H, d, J 8.8, Ar-oH), 8.24 (1 H, s, 3-H), 8.39 (1 H, s, CH-Ar), 12.35 (1 H, br s, 4-NH), 13.60 (1 H, br, 1-NH) |
3g![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3190, 3090 (NH) | 8.09 (2 H, d, J 8.8, Ar-oH), 8.32 (2 H, d, J 8.8, Ar-mH), 8.35 (1 H, s, 3-H), 8.46 (1 H, s, CH-Ar), 12.78 (1 H, br s, 4-NH), 13.90 (1 H, br, 1-NH) |
| 8b | 3240, 3180, 3140 (NH) | 7.26–7.83 (10 H, m, Ph-H × 2), 8.19 (1 H, s, 6-CH-Ar), 8.24 (1 H, s, 3-H), 8.32 (1 H, s, 4-CH-Ar), 10.81 (1 H, br s, 6-NH), 11.78 (1 H, br, 4-NH), 13.07 (1 H, br, 1-NH) |
| 8c | 3250, 3170, 3130 (NH) | 7.27 (2 H, dd, JH,H 8.8, JH,F 9.1, 6-Ar-mH), 7.32 (2 H, dd, JH,H 8.8, JH,F 9.1, 4-Ar-mH), 7.74 (2 H, dd, JH,H 8.8, JH,F 5.9, 6-Ar-oH), 7.87 (2 H, dd, JH,H 8.8, JH,F 5.8, 4-Ar-oH), 8.20 (1 H, s, 6-CH-Ar), 8.26 (1 H, s, 3-H), 8.33 (1 H, s, 4-CH-Ar), 10.93 (1 H, br s, 6-NH), 11.80 (1 H, br, 4-NH), 12.90 (1 H, br, 1-NH) |
| 8d | 3250, 3180, 3120 (NH) | 7.45 (2 H, d, J 8.4, 6-Ar-mH), 7.51 (2 H, d, J 8.5, 4-Ar-mH), 7.74 (2 H, d, J 8.4, 6-Ar-oH), 7.80 (2 H, d, J 8.5, 4-Ar-oH), 8.17 (1 H, s, 6-CH-Ar), 8.28 (1 H, s, 3-H), 8.32 (1 H, s, 4-CH-Ar), 10.95 (1 H, br, 6-NH), 11.80 (1 H, br, 4-NH), 13.00 (1 H, br, 1-NH) |
| 8f | 3250, 3170, 3140 (NH) | 3.75 (3 H, s, 6-OCH3), 3.81 (3 H, s, 4-OCH3), 6.95 (2 H, d, J 8.5, 6-Ar-mH), 7.06 (2 H, d, J 8.8, 4-Ar-mH), 7.60 (2 H, d, J 8.5, 6-Ar-oH), 7.77 (2 H, d, J 8.8, 4-Ar-oH), 8.12 (1 H, s, 6-CH-Ar), 8.20 (1 H, s, 3-H), 8.24 (1 H, s, 4-CH-Ar), 10.59 (1 H, br s, 6-NH), 11.66 (1 H, br, 4-NH), 12.83 (1 H, br, 1-NH) |
 | ||
| Scheme 1 Reagents and conditions: i, anh. NH2NH2, 2-methoxyethanol, 0 °C, 0.5 h; ii, RCHO, DMF, r.t., 10 h; iii, conc. HCl reflux, 1 h; iv, 10% HCl, reflux, 3 h; v, P2S5, pyridine, reflux, 2 h; vi, 50% ethanolic NH2NH2, reflux, 10 min; vii, anh. NH2NH2, 2-methoxyethanol, 100 °C, 5 h; viii, 80% aq. NH2NH2, 80–90 °C, 4 h; ix, RCHO, DMF, r.t., 10 h; x, urea, 2-ethoxyethanol, reflux, 5 h; xi, urea, 2-ethoxyethanol, reflux, 10 h; xii, urea, 2-ethoxyethanol, 36 h. | ||
On the other hand, heating compound 1 with excess anhydrous hydrazine at 100 °C or heating compound 2 with excess 80% hydrazine hydrate at 80–90 °C afforded the 4,6-dihydrazino derivative 7 in good yields. Subsequent reaction of compound 7 with appropriate aldehydes (3.0 equiv.) in DMF at room temperature gave the corresponding hydrazones 8b–d,f in excellent yields as shown in Tables 1 and 2. Next, in an attempt to convert the 6-chloro-4-hydrazino compound 2 to the 6-oxo-4-hydrazino derivative 6, compound 2 was reacted with urea (4.0 equiv.) in 2-ethoxyethanol under reflux for 5 hours. However, owing to the stability of the chloro group towards hydroxy substitution by urea or alkali, the intended compound 6 was not obtained, but 4-carbamoylhydrazino-6-chloro-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidine 9, which resulted from carbamoylation of the hydrazino group at the 4-position, was formed in 60% yield. Heating under reflux the product 9 with urea (3.0 equiv.) in 2-ethoxyethanol afforded the desired tricyclic compound, 3-amino-7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H
]pyrimidine 9, which resulted from carbamoylation of the hydrazino group at the 4-position, was formed in 60% yield. Heating under reflux the product 9 with urea (3.0 equiv.) in 2-ethoxyethanol afforded the desired tricyclic compound, 3-amino-7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 10, in 18% yield. This compound 10 (29% yield) was also obtained by prolonged heating of 2 with urea under the same reaction conditions as mentioned above.
)-one 10, in 18% yield. This compound 10 (29% yield) was also obtained by prolonged heating of 2 with urea under the same reaction conditions as mentioned above.
All new compounds 2, 3 and 6–10 exhibited satisfactory elemental combustion analyses except for 2 and 6 and FAB-MS, IR and 1H NMR spectral data consistent with the structures. In particular, the structure of the product 10 was confirmed by the presence of a two-proton broad singlet signal at δ 7.94 and one-proton signals at δ 12.27 and 13.10 in the 1H NMR spectrum attributable to the amino and imino groups and by the presence of peaks at 3370 (νas NH), 3260 (νs NH), 1720 (ν C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) and 1685 (δ NH) cm−1 in the IR spectrum attributable to amino and carbonyl groups. It was clarified that the substitution reaction of the chloro group by hydroxy was difficult in the pyrazolopyrimidine ring 2, while in the pyrazolotriazolopyrimidine ring 10 it was easy.
O) and 1685 (δ NH) cm−1 in the IR spectrum attributable to amino and carbonyl groups. It was clarified that the substitution reaction of the chloro group by hydroxy was difficult in the pyrazolopyrimidine ring 2, while in the pyrazolotriazolopyrimidine ring 10 it was easy.
The 4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 6 as noted above was a versatile intermediate for the preparation of the 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidine ring system. Thus the hydrazinopyrazolopyrimidine 6 could be converted to the hydrazones 11b, e–r (63–95% yields) by reaction with an appropriate aldehyde (1.5 equiv.) in DMF at room temperature (Scheme 2 and Tables 3 and 4). The hydrazones 11b, e–p, r were subsequently cyclised to the corresponding 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H
)-one 6 as noted above was a versatile intermediate for the preparation of the 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidine ring system. Thus the hydrazinopyrazolopyrimidine 6 could be converted to the hydrazones 11b, e–r (63–95% yields) by reaction with an appropriate aldehyde (1.5 equiv.) in DMF at room temperature (Scheme 2 and Tables 3 and 4). The hydrazones 11b, e–p, r were subsequently cyclised to the corresponding 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones 12b, e–p, r by heating with 70% nitric acid (1.2 equiv.) at 100 °C in 60–91% yields (Method A) (Tables 5 and 6). In the case of compound 11q possessing a 4-hydroxybenzylidenehydrazino group as the substituent at the 4-position, the 3-(4-hydroxy-3-nitrophenyl) derivative 12s was obtained by oxidative cyclisation–nitration in 52% yield. Oxidative cyclisation was also accomplished by heating compounds 11e, g, h, k, m–o, q with diethyl azodicarboxylate (DEAD) (3–7 equiv.) under reflux in 50–67% yields (Method B). Moreover, the 3-alkyl derivatives 12a–d were synthesised by treatment of compound 6 with an appropriate trialkyl orthoester (5.0 equiv.) in trifluoroacetic acid at room temperature (Method C) or heating compound 6 with trialkyl orthoesters (3.0 equiv.) in DMF at 100 °C (Method D) in 54–83% yields. In the light of this multiple step synthesis, a one-pot oxidative cyclisation starting from the 6-chloro-4-hydrazones 3a–g would be attractive. Indeed, heating the hydrazones 3a–g with 70% nitric acid (5.0 equiv.) in DMF at 100 °C afforded the desired 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H
)-ones 12b, e–p, r by heating with 70% nitric acid (1.2 equiv.) at 100 °C in 60–91% yields (Method A) (Tables 5 and 6). In the case of compound 11q possessing a 4-hydroxybenzylidenehydrazino group as the substituent at the 4-position, the 3-(4-hydroxy-3-nitrophenyl) derivative 12s was obtained by oxidative cyclisation–nitration in 52% yield. Oxidative cyclisation was also accomplished by heating compounds 11e, g, h, k, m–o, q with diethyl azodicarboxylate (DEAD) (3–7 equiv.) under reflux in 50–67% yields (Method B). Moreover, the 3-alkyl derivatives 12a–d were synthesised by treatment of compound 6 with an appropriate trialkyl orthoester (5.0 equiv.) in trifluoroacetic acid at room temperature (Method C) or heating compound 6 with trialkyl orthoesters (3.0 equiv.) in DMF at 100 °C (Method D) in 54–83% yields. In the light of this multiple step synthesis, a one-pot oxidative cyclisation starting from the 6-chloro-4-hydrazones 3a–g would be attractive. Indeed, heating the hydrazones 3a–g with 70% nitric acid (5.0 equiv.) in DMF at 100 °C afforded the desired 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones 12e, g, h, k, m, n, r accompanied by hydrolytic dechlorination in 60–85% yields (Method E).
)-ones 12e, g, h, k, m, n, r accompanied by hydrolytic dechlorination in 60–85% yields (Method E).
| (Found (%) (Required) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound (Formula) | Reaction temp/°C | Yield (%) | Mp/°C | Recrystn. solvent![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a (Rf, solvent system a (Rf, solvent system![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b) b) |
C | H | N | m/z MH+ |
| a All compounds 11 were obtained as colourless powdery crystals except for 11m, r (pale yellow). b Solvent systems: (A) AcOEt–EtOH (4∶1 v/v), (B) AcOEt–EtOH (9∶1 v/v), (C) AcOEt–n-hexane–AcOH (8∶4∶1 v/v). | ||||||||
| 11b | r.t. | 89 | >300 | EtOH–DMF | 43.4 | 4.2 | 43.65 | 193 |
| C7H8N6O | (0.47, A) | (43.75) | (4.2) | (43.7) | ||||
| 11e | r.t. | 80 | >300 | EtOH–DMF | 55.8 | 7.2 | 30.3 | 277 |
| C13H20N6O·1/5 H2O | (0.65, A) | (55.8) | (7.35) | (30.0) | ||||
| 11f | r.t. | 63 | >300 | EtOH–DMF | 60.3 | 7.4 | 26.3 | 317 |
| C16H24N6O | (0.47, B) | (60.7) | (7.65) | (26.6) | ||||
| 11g | r.t. | 85 | >300 | water–DMF | 55.8 | 4.1 | 32.8 | 255 |
| C12H10N6O·1/5 H2O | (0.60, A) | (55.9) | (4.1) | (32.6) | ||||
| 11h | r.t. | 74 | >300 | water–DMF | 52.2 | 3.6 | 30.5 | 273 |
| C12H9FN6O·1/4 H2O | (0.64, A) | (52.1) | (3.5) | (30.4) | ||||
| 11i | 40 | 76 | >300 | EtOH–DMF | 49.5 | 3.5 | 28.7 | 289/291 |
| C12H9ClN6O | (0.64, A) | (49.9) | (3.1) | (29.1) | ||||
| 11j | 40 | 95 | >300 | EtOH–DMF | 49.3 | 3.4 | 28.9 | 289/291 |
| C12H9ClN6O·1/5 H2O | (0.65, A) | (49.3) | (3.2) | (28.75) | ||||
| 11k | r.t. | 95 | >300 | water–DMF | 49.2 | 3.4 | 28.7 | 289/291 |
| C12H9ClN6O·1/5 H2O | (0.63, A) | (49.3) | (3.2) | (28.75) | ||||
| 11l | r.t. | 83 | >300 | EtOH–DMF | 43.3 | 3.1 | 24.9 | 333/335 |
| C12H9BrN6O | (0.65, A) | (43.3) | (2.7) | (25.2) | ||||
| 11m | r.t. | 93 | >300 | DMF | 57.4 | 4.5 | 31.1 | 269 |
| C13H12N6O·1/5 H2O | (0.67, A) | (57.4) | (4.6) | (30.9) | ||||
| 11n | r.t. | 87 | >300 | water–DMF | 54.4 | 4.3 | 29.2 | 285 |
| C13H12N6O2·1/5 H2O | (0.62, A) | (54.2) | (4.3) | (29.2) | ||||
| 11o | 40 | 85 | >300 | water–DMF | 52.1 | 3.6 | 27.7 | 299 |
| C13H10N6O3 | (0.67, A) | (52.35) | (3.4) | (28.2) | ||||
| 11p | 40 | 80 | >300 | water–DMF | 52.6 | 3.4 | 28.2 | 299 |
| C13H10N6O3 | (0.64, C) | (52.35) | (3.4) | (28.2) | ||||
| 11q | 40 | 85 | >300 | EtOH–DMF | 53.2 | 4.0 | 30.7 | 271 |
| C12H10N6O2 | (0.64, A) | (53.3) | (3.7) | (31.1) | ||||
| 11r | r.t. | 88 | >300 | EtOH–DMF | 45.45 | 3.5 | 30.8 | 300 |
| C12H9N7O3·H2O | (0.60, A) | (45.4) | (3.5) | (30.9) | ||||
| Compound | ν max(Nujol)/cm−1 | δ H [200 MHz; (CD3)2SO; Me4Si] |
|---|---|---|
| a This compound was measured at 60 MHz. | ||
| 11b | 3175, 3130, 3070 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
2.01 (3 H, d, J 5.4, CHCH3), 7.69 (1 H, q, J 5.4, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) CH3), 8.35 (1 H, s, 3-H), 10.40 (1 H, br, 4-NH), 10.80 (1 H, br s, 7-NH), 12.85 (1 H, br, 1-NH) CH3), 8.35 (1 H, s, 3-H), 10.40 (1 H, br, 4-NH), 10.80 (1 H, br s, 7-NH), 12.85 (1 H, br, 1-NH) |
| 11e | 3175, 3135, 3070 (NH); 1710 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
0.86 (3 H, t, J 6.4, CHCH2CH2[CH2]4CH3), 1.29 (8 H, br s, CHCH2CH2[CH2]4CH3), 1.44–1.66 (2 H, m, CHCH2CH2[CH2]4CH3), 2.24–2.42 (2 H, m, CHCH2CH2[CH2]4CH3), 8.05 (1 H, t, J 5.4, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) CH2CH2[CH2]4CH3), 8.28 (1 H, s, 3-H), 10.22 (1 H, br, 4-NH), 10.82 (1 H, br s, 7-NH), 12.95 (1 H, br, 1-NH) CH2CH2[CH2]4CH3), 8.28 (1 H, s, 3-H), 10.22 (1 H, br, 4-NH), 10.82 (1 H, br s, 7-NH), 12.95 (1 H, br, 1-NH) |
| 11f | 3175, 3135, 3070 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
1.28 (10 H, br s, CHCH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 1.44–1.64 (2 H, m, CHCH2CH2[CH2]5CH2CH CH2), 1.44–1.64 (2 H, m, CHCH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 1.90–2.08 (2 H, m, CHCH2CH2[CH2]5CH2CH CH2), 1.90–2.08 (2 H, m, CHCH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 2.22–2.42 (2 H, m, CHCH2CH2[CH2]5CH2CH CH2), 2.22–2.42 (2 H, m, CHCH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 4.86–5.06 (2 H, m, CHCH2CH2[CH2]5CH2CH CH2), 4.86–5.06 (2 H, m, CHCH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 5.64–5.90 (1 H, m, CHCH2CH2[CH2]5CH2CH CH2), 5.64–5.90 (1 H, m, CHCH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 7.60–7.72 (1 H, m, CH CH2), 7.60–7.72 (1 H, m, CH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) CH2CH2[CH2]5CH2CH CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 8.28 (1H, s, 3-H), 10.28 (1 H, br, 4-NH), 10.82 (1 H, br s, 7-NH), 12.92 (1 H, br, 1-NH) CH2), 8.28 (1H, s, 3-H), 10.28 (1 H, br, 4-NH), 10.82 (1 H, br s, 7-NH), 12.92 (1 H, br, 1-NH) |
| 11g | 3200, 3120, 3080 (NH); 1680 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.40–7.53 (3 H, m, Ph-m,pH), 7.80–7.90 (2 H, m, Ph-oH), 8.40 (1 H, s, 3-H), 8.46 (1 H, s, CH-Ar), 10.40 (1 H, br, 4-NH), 11.01 (1 H, br, 7-NH), 13.10 (1 H, br, 1-NH) |
| 11h | 3180, 3140, 3070 (NH); 1680 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.30 (2 H, dd, JH,H 8.8, JH,F 9.0, Ar-mH), 7.30 (2 H, dd, JH,H 8.8, JH,F 5.8, Ar-oH), 8.40 (1 H, s, 3-H), 8.45 (1H, s, CH-Ar), 10.40 (1 H, br, 4-NH), 11.00 (1 H, br, 7-NH), 13.09 (1 H, br, 1-NH) |
11i![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3200, 3120, 3080 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.33–7.60 (3 H, m, 3′-H, 4′-H and 5′-H), 8.12–8.28 (1 H, m, 6′-H), 8.48 (1H, s, 3-H), 8.72 (1 H, s, CH-Ar), 10.35 (1 H, br s, 4-NH), 11.10 (1 H, br, 7-NH), 13.40 (1 H, br, 1-NH) |
| 11j | 3170, 3140, 3060 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.40–7.54 (2 H, m, 4′-H and 5′-H), 7.80–7.92 (2 H, m, 2′-H and 6′-H), 8.40 (1 H, s, 3-H), 8.43 (1 H, s, CH-Ar), 10.50 (1 H, br s, 4-NH), 11.01 (1 H, br s, 7-H), 13.10 (1 H, br s, 1-NH) |
| 11k | 3170, 3140, 3070 (NH); 1680 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.48 (2 H, d, J 8.6, Ar-mH), 7.87 (2 H, d, J 8.6, Ar-oH), 8.37 (1 H, s, 3-H), 8.45 (1 H, s, CH-Ar), 10.50 (1 H, br, 4-NH), 11.00 (1 H, br, 7-NH), 13.14 (1 H, br, 1-NH) |
| 11l | 3170, 3140, 3060 (NH); 1685 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.66 (2 H, d, J 8.6, Ar-mH), 7.80 (2 H, d, J 8.6, Ar-oH), 8.38 (1H, s, 3-H), 9.44 (1 H, s, CH-Ar), 10.50 (1 H, br, 4-NH), 11.00 (1 H, br s, 7-NH), 13.11 (1 H, br, 1-NH) |
| 11m | 3170, 3140, 3060 (NH); 1685 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
2.36 (3 H, s, CH3), 7.28 (2 H, d, J 7.8, Ar-mH), 7.72 (2 H, d, J 7.8, Ar-oH), 8.35 (1 H, s, 3-H), 8.44 (1 H, s, CH-Ar), 10.45 (1 H, br, 4-NH), 11.00 (1 H, br, 7-NH), 13.10 (1 H, br, 1-NH) |
11n![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3180, 3140, 3080 (NH); 1680 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
3.80 (3 H, s, OCH3), 6.74 (2 H, d, J 8.5, Ar-mH), 7.58 (2 H, d, J 8.5, Ar-oH), 7.94 (1 H, s, 3-H), 7.99 (1 H, s, CH-Ar), 10.06 (1 H, br s, 4-NH), 10.92 (1 H, br s, 7-NH), 12.90 (1 H, br s, 1-NH) |
| 11o | 3180, 3140, 3070 (NH); 1655 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
6.09 (2 H, s, OCH2O), 7.00 (1 H, d, J5′,6′ 8.2, 5′-H), 7.27 (1 H, d, J5′,6′ 8.2, J2′,6′ 1.6, 6′-H), 7.45 (1 H, d, J2′,6′ 1.6, 2′-H), 8.30 (1 H, s, 3-H), 8.45 (1 H, s, CH-Ar), 10.35 (1 H, br, 4-NH), 11.03 (1 H, br s, 7-NH), 13.04 (1 H, br, 1-NH) |
11p![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3165, 3120, 3050 (NH); 1650, 1630 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
8.00 (4 H, br, Ar-H), 8.50 (2 H, s, 3-H and CH-Ar), 11.25 (1 H, br, 4-NH), 11.95 (1 H, br, 7-NH), 12.35 (1 H, br, COOH![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ), 13.55 (1 H, br, 1-NH) ), 13.55 (1 H, br, 1-NH) |
| 11q | 3160, 3140, 3050 (NH); 1640 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
6.84 (2 H, d, J 8.4, Ar-mH), 7.65 (2 H, d, J 8.4, Ar-oH), 8.26 (1 H, s, 3-H), 8.41 (1 H, s, CH-Ar), 9.67 (1 H, br s, OH), 10.86 (1 H, br s, 4-NH), 11.02 (1 H, br s, 7-NH), 12.90 (1 H, br, 1-NH) |
| 11r | 3165, 3120, 3080 (NH); 1690 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
8.10 (1 H, d, J 8.8, Ar-oH), 8.30 (1 H, d, J 8.8, Ar-mH), 8.50 (1 H, s, 3-H), 8.52 (1 H, s, CH-Ar), 10.65 (1 H, br, 4-NH), 11.11 (1 H, br, 7-NH), 13.21 (1 H, br, 1-NH) |
Reaction conditions![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
(Found (%) (Required) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound (Formula) | Method | Temp/°C | Time/h | Yield![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a (%) a (%) |
Mp/°C | Recrystn. solvent![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b (Rf, solvent system b (Rf, solvent system![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c) c) |
C | H | N | m/z MH+ |
| a The reaction conditions and yields depend on the particular method. b All compounds 12 were obtained as colourless powdery crystals except for 12b, h, k, l, (colourless needles) and 12r, s (yellow powder). c Solvent systems: (A) AcOEt–EtOH (4∶1 v/v), (B) AcOEt–EtOH (9∶1 v/v), (C) AcOEt–n-hexane–AcOH (8∶4∶1 v/v). | ||||||||||
| 12a | (C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
r.t. | 1 | 66 | >300 | DMF | 39.9 | 2.7 | 46.5 | 177 |
| C6H4N6O·1/4 H2O | (D) | 100 | 1 | 65 | (0.32, A) | (39.9) | (2.5) | (46.5) | ||
| 12b | (A) | 100 | 2.5 | 64 | >300 | EtOH–DMF | 43.5 | 3.5 | 43.7 | 191 |
| C7H6N6O·1/5 H2O | (C![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
r.t | 1 | 83 | (0.38, A) | (43.4) | (3.3) | (43.4) | ||
| (D) | 100 | 1 | 55 | |||||||
| 12c | (D) | 100 | 1 | 54 | >300 | EtOH–DMF | 46.8 | 4.2 | 41.4 | 205 |
| C8H8N6O | (0.45, A) | (47.1) | (3.95) | (41.2) | ||||||
| 12d | (D) | 100 | 1 | 55 | >300 | EtOH–DMF | 51.1 | 5.5 | 36.0 | 233 |
| C10H12N6O·1/5 H2O | (0.55, A) | (50.9) | (5.3) | (35.6) | ||||||
| 12e | (A) | 100 | 3 | 77 | >300 | EtOH–DMF | 55.7 | 6.7 | 30.2 | 275 |
| C13H18N6O·1/4 H2O | (B) | reflux | 7 | 60 | (0.64, A) | (56.0) | (6.7) | (30.1) | ||
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 1 | 75 | |||||||
| 12f | (A) | 100 | 2.5 | 67 | >300 | EtOH–DMF | 60.3 | 7.35 | 26.3 | 315 |
| C16H22N6O·1/4 H2O | (0.34, B) | (60.3) | (7.1) | (26.35) | ||||||
| 12g | (A) | 100 | 1 | 91 | >300 | water–DMF | 55.9 | 3.45 | 32.75 | 253 |
| C12H8N6O/1/4 H2O | (B) | reflux | 5 | 60 | (0.60, A) | (56.1) | (3.3) | (32.7) | ||
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 1 | 85 | |||||||
| 12h | (A) | 100 | 1 | 91 | >300 | EtOH–DMF | 52.6 | 2.9 | 30.65 | 271 |
| C12H7FN6O·1/4 H2O | (B) | reflux | 8 | 65 | (0.62, A) | (52.5) | (2.75) | (30.6) | ||
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 3 | 72 | |||||||
| 12i | (A) | 100 | 2 | 70 | >300 | water–DMF | 50.0 | 2.8 | 28.9 | 287/289 |
| C12H7ClN6O | (0.63, A) | (50.3) | (2.5) | (29.3) | ||||||
| 12j | (A) | 100 | 2 | 76 | >300 | water–DMF | 49.9 | 2.8 | 29.2 | 287/289 |
| C12H7ClN6O·1/5 H2O | (0.64, A) | (49.65) | (2.6) | (28.95) | ||||||
| 12k | (A) | 100 | 2 | 90 | >300 | water–DMF | 49.7 | 2.8 | 29.2 | 287/289 |
| C12H7ClN6O·1/5 H2O | (B) | reflux | 8 | 67 | (0.63, A) | (49.65) | (2.6) | (28.95) | ||
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 1 | 71 | |||||||
| 12l | (A) | 100 | 5 | 74 | >300 | EtOH–DMF | 43.2 | 2.5 | 24.9 | 331/333 |
| C12H7BrN6O·1/5 H2O | (0.64, A) | (43.1) | (2.2) | (25.1) | ||||||
| 12m | (A) | 100 | 9 | 60 | >300 | water–DMF | 58.0 | 4.0 | 31.3 | 267 |
| C13H10N6O·1/5 H2O | (B) | reflux | 9 | 54 | (0.67, A) | (57.9) | (3.9) | (31.1) | ||
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 3 | 67 | |||||||
| 12n | (A) | 100 | 3 | 88 | >300 | water–DMF | 54.5 | 3.9 | 29.3 | 283 |
| C13H10N6O2·1/4 H2O | (B) | reflux | 9 | 57 | (0.60, A) | (54.45) | (3.7) | (29.3) | ||
(E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 3 | 61 | |||||||
| 12o | (A) | 100 | 1 | 81 | >300 | EtOH–DMF | 52.2 | 3.1 | 28.0 | 297 |
| C13H8N6O3·1/4 H2O | (B) | reflux | 9 | 55 | (0.62, A) | (51.9) | (2.85) | (27.9) | ||
| 12p | (A) | 100 | 1.5 | 69 | >300 | EtOH–DMF | 51.8 | 3.2 | 27.6 | 297 |
| C13H8N6O3·1/3 H2O | (0.64, C) | (51.7) | (2.9) | (27.8) | ||||||
| 12q | (B) | reflux | 9 | 50 | >300 | EtOH–DMF | 52.8 | 2.9 | 31.0 | 269 |
| C12H8N6O2·1/4 H2O | (0.51, A) | (52.85) | (3.1) | (30.8) | ||||||
| 12r | (A) | 100 | 5 | 60 | >300 | EtOH–DMF | 48.1 | 2.8 | 32.2 | 298 |
| C12H7N7O3·1/4 H2O | (E![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ) ) |
100 | 5 | 60 | (0.60, A) | (47.8) | (2.5) | (32.5) | ||
| 12s | (A) | 100 | 1 | 52 | >300 | DMF | 45.4 | 2.6 | 30.6 | 314 |
| C12H7N7O4·1/4 H2O | (0.46, A) | (45.4) | (2.4) | (30.9) | ||||||
| Compound | ν max(Nujol)/cm−1 | δ H [200 MHz; (CD3)2SO; Me4Si] |
|---|---|---|
| a This compound was measured at 60 MHz. | ||
| 12a | 3120, 3030 (NH); 1740 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
8.34 (1 H, s, 9-H), 8.62 (1 H, s, 3-H), 12.58 (1 H, br s, 6-NH), 13.60 (1 H, br s, 7-NH) |
| 12b | 3110, 3050 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
2.40 (3 H, s, CH3), 8.54 (1 H, s, 9-H), 12.46 (1 H, br s, 6-NH), 13.57 (1 H, br s, 7-NH) |
| 12c | 3100, 3060 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
1.29 (3 H, t, J 7.6, CH2CH3), 2.77 (2 H, q, J 7.6, CH2CH3), 8.57 (1 H, s, 9-H), 12.43 (1 H, br s, 6-NH), 13.55 (1 H, br s, 7-NH) |
| 12d | 3090, 3060 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
0.92 (3 H, t, J 7.6, CH2CH2CH2CH3), 1.36 (2 H, sextet, J 7.6, CH2CH2CH2CH3), 1.72 (2 H, quintet, J 7.6, CH2CH2CH2CH3), 2.74 (2 H, t, J 7.6, CH2CH2CH2CH3), 8.57 (1 H, s, 9-H), 12.44 (1 H, br s, 6-NH), 13.55 (1 H, br s, 7-NH) |
12e![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3110, 3070 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
0.86 (3 H, t, J 6.5, CH2CH2[CH2]4CH3), 1.30 (8 H, br s, CH2CH2[CH2]4CH3), 1.50–1.80 (2 H, m, CH2CH2[CH2]4CH3), 2.50–2.95 (2 H, m, CH2CH2[CH2]4CH3), 8.53 (1 H, s, 9-H), 12.41 (1 H, br s, 6-NH), 13.50 (1 H, br, 7-NH) |
| 12f | 3150, 3070 (NH); 1710 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
1.28 (10 H, br s, CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 1.62–1.80 (2 H, m, CH2CH2[CH2]5CH2CH CH2), 1.62–1.80 (2 H, m, CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 1.92–2.06 (2 H, m, CH2CH2[CH2]5CH2CH CH2), 1.92–2.06 (2 H, m, CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 2.72 (2 H, t, J 7.3, CH2CH2[CH2]5CH2CH CH2), 2.72 (2 H, t, J 7.3, CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 4.87–5.04 (2 H, m, CH2CH2[CH2]5CH2CH CH2), 4.87–5.04 (2 H, m, CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 5.66–5.89 (1 H, m, CH2CH2[CH2]5CH2CH CH2), 5.66–5.89 (1 H, m, CH2CH2[CH2]5CH2CH![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2), 8.57 (1 H, s, 9-H), 12.43 (1 H, br s, 6-NH), 13.55 (1 H, br s, 7-NH) CH2), 8.57 (1 H, s, 9-H), 12.43 (1 H, br s, 6-NH), 13.55 (1 H, br s, 7-NH) |
12g![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3150, 3050 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.40–7.70 (3 H, m, Ph-m,pH), 7.90–8.35 (2 H, m, Ph-oH), 8.68 (1 H, s, 9-H), 12.60 (1 H, br s, 6-NH), 13.60 (1 H, br, 7-NH) |
12h![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3110, 3090 (NH); 1710 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.37 (2 H, dd, JH,H 8.8, JH,F 9.1, Ar-mH), 8.22 (2 H, dd, JH,H 8.8, JH,F 5.9, Ar-oH), 8.66 (1 H, s, 9-H), 12.59 (1 H, br s, 6-NH), 13.65 (1 H, br, 7-NH) |
| 12i | 3150, 3070 (NH); 1710 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.44–7.78 (3 H, m, 3′-H, 4′-H and 5′-H), 7.95–8.09 (1 H, m, 6′-H), 8.69 (1 H, s, 9-H), 12.67 (1 H, br s, 6-NH), 13.58 (1 H, br, 7-NH) |
12j![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3150, 3100 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.54–7.63 (2 H, m, 4′-H and 5′-H), 7.95–8.25 (2 H, m, 2′-H and 6′-H), 8.67 (1 H, s, 9-H), 12.62 (1 H, br s, 6-NH), 13.60 (1 H, br s, 7-NH) |
| 12k | 3160, 3100 (NH); 1700 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.62 (2 H, d, J 8.6, Ar-mH), 8.17 (2 H, d, J 8.6, Ar-oH), 8.70 (1 H, s, 9-H), 12.62 (1 H, br s, 6-NH), 13.66 (1 H br s, 7-NH) |
| 12l | 3150, 3060 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.76 (2 H, d, J 8.6, Ar-mH), 8.10 (2 H, d, J 8.6, Ar-oH), 8.69 (1 H, s, 9-H), 12.62 (1 H, br s, 6-NH), 13.65 (1 H, br s, 7-NH) |
| 12m | 3180, 3100 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
2.39 (3 H, s, CH3), 7.35 (2 H, d, J 8.0, Ar-mH), 8.05 (2 H, d, J 8.0, Ar-oH), 8.66 (1 H, s, 9-H), 12.54 (1 H, br s, 6-NH), 13.61 (1 H, br s, 7-NH) |
12n![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3160, 3100 (NH); 1730 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
3.85 (3 H, s, OCH3), 7.10 (2 H, d, J 8.8, Ar-mH), 8.12 (2 H, d, J 8.8, Ar-oH), 8.64 (1 H, s, 9-H), 12.60 (1 H, br s, 6-NH), 13.50 (1 H, br, 7-NH) |
| 12o | 3160, 3050 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
6.13 (2 H, s, OCH2O), 7.08 (1 H, d, J5′,6′ 8.1, 5′-H), 7.58 (1 H, d, J2′,6′ 1.6, 2′-H), 7.72 (1 H, d, J5′,6′ 8.1, J2′,6′ 1.6, 6′-H), 8.68 (1 H, s, 9-H), 12.55 (1 H, br s, 6-NH), 13.62 (1 H, br s, 7-NH) |
12p![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3160, 3090 (NH); 1700, 1660 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
8.10 (2 H, d, J 8.8, Ar-mH), 8.32 (2 H, d, J 8.8, Ar-oH), 8.69 (1 H, s, 9-H), 12.70 (1 H, br s, 6-NH), 13.50 (2 H, br, 7-NH and COOH) |
| 12q | 3160, 3050 (NH); 1718 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
6.90 (2 H, d, J 8.8, Ar-mH), 7.99 (2 H, d, J 8.8, Ar-oH), 8.66 (1 H, s, 9-H), 9.94 (1 H, s, OH), 12.51 (1 H, br s, 6-NH), 13.60 (1 H, br, 7-NH) |
12r![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
3170, 3100 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
8.39 (4 H, br s, Ar-H), 8.69 (1 H, s, 9-H), 12.70 (1 H, br s, 6-NH), 13.60 (1 H, br 7-NH) |
| 12s | 3160, 3080 (NH); 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) O) |
7.31 (1 H, d, J5′,6′ 8.8, 5′-H), 8.28 (1 H, dd, J5′,6′ 8.8. J2′,6′ 2.2, 6′-H), 8.60 (1 H, d, J2′,6′ 2.2, 2′-H), 8.70 (1 H, s, 9-H), 11.59 (1 H, s, OH), 12.62 (1 H, br s, 6-NH), 13.65 (1 H, br s, 7-NH) |
 | ||
| Scheme 2 Reagents and conditions: i, RCHO, DMF, r.t. or 40 °C, 10 h; ii, 70% HNO3, DMF, 100 °C, 1–9 h; iii, DEAD, DMF, reflux, 5–9 h; iv, RC(OEt)3, TFA, r.t., 1 h; v, RC(OEt)3 or RC(OMe)3, DMF, 100 °C, 1 h. | ||
All new compounds 11 and 12 exhibited satisfactory elemental combustion analyses and FAB MS, IR and 1H NMR spectral data consistent with the structures as indicated in Tables 3–6.
Xanthine oxidase inhibitory results
The novel pyrazolopyrimidines 2, 3, 6, 8 and 11 and pyrazolotriazolopyrimidines 12 prepared in this study were tested as inhibitors of bovine milk xanthine oxidase in a similar assay method![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 14 as previously reported. The inhibition (%) and IC50 (μM) values of the compounds tested against bovine milk xanthine oxidase are shown in Table 7. Thus the introduction of both an aryl aldehyde hydrazone at the 4-position and an oxo group at the 6-position of the 1H-pyrazolo[3,4-d
14 as previously reported. The inhibition (%) and IC50 (μM) values of the compounds tested against bovine milk xanthine oxidase are shown in Table 7. Thus the introduction of both an aryl aldehyde hydrazone at the 4-position and an oxo group at the 6-position of the 1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidine ring led to markedly better activities in xanthine oxidase inhibition, these compounds being two orders of magnitude more active than allopurinol: IC50 values for 11g–k and 11m–q were ca. 0.08–0.4 μM, whereas that for allopurinol was 24.3 μM. Most of the pyrazolotriazolopyrimidines 12a–s showed potent inhibitory activities, being two or three orders of magnitude more active than allopurinol. Of these compounds, 12k (R = 4-ClC6H4) was the most active; it showed a 760-fold (IC50 = 0.032 μM) more potent bovine milk XO inhibitory activity than that of allopurinol.
]pyrimidine ring led to markedly better activities in xanthine oxidase inhibition, these compounds being two orders of magnitude more active than allopurinol: IC50 values for 11g–k and 11m–q were ca. 0.08–0.4 μM, whereas that for allopurinol was 24.3 μM. Most of the pyrazolotriazolopyrimidines 12a–s showed potent inhibitory activities, being two or three orders of magnitude more active than allopurinol. Of these compounds, 12k (R = 4-ClC6H4) was the most active; it showed a 760-fold (IC50 = 0.032 μM) more potent bovine milk XO inhibitory activity than that of allopurinol.
| Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|
| Compound No. | 10 | 3 | 1 | 0.3 | 0.1 | 0.03 | IC50/μM |
| a 30 μM: 53.9%, 100 μM: 63.9%. b This value is inaccurate because of insolubility in DMSO. c 0.01 μM: 12.6%. d 0.01 μM: 9.6%. e 0.01 μM: 30.4%, 0.003 μM, 16.4%. f 0.01 μM: 37.3%. g 0.01 μM: 28.2%, 0.003 μM: 13.6%. h 0.01 μM: 29.0%. i Allo: allopurinol. | |||||||
| 2 | 21.4 | 11.8 | 8.3 | >10 | |||
| 3b | 7.6 | >10 | |||||
| 3c | 21.0 | 16.0 | 11.5 | >10 | |||
| 3d | 12.0 | 9.5 | 9.4 | >10 | |||
| 3e | 14.2 | 13.8 | 10.8 | >10 | |||
| 3f | 41.0 | >10 | |||||
4![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) a a |
39.8 | 22.1 | |||||
| 6 | 24.1 | >10 | |||||
| 8b | 7.2 | >10 | |||||
| 8c | 16.3 | >10 | |||||
| 8d | 12.4 | >10 | |||||
| 8f | 17.2 | >10 | |||||
| 11b | 58.6 | 45.0 | 34.8 | 15.8 | 6.9 | 6.2 | 4.670 |
| 11e | 71.7 | 69.2 | 60.6 | 44.6 | 25.8 | 13.3 | 0.450 |
| 11f | 53.3 | 36.5 | 22.4 | 13.0 | 9.7 | 4.6 | 7.894 |
| 11g | 75.6 | 73.0 | 66.3 | 49.8 | 29.9 | 0.305 | |
| 11h | 68.5 | 69.9 | 63.6 | 47.0 | 29.7 | 0.373 | |
| 11i | 68.9 | 69.6 | 68.5 | 64.4 | 53.8 | 36.3 | 0.077 |
| 11j | 67.6 | 66.4 | 63.4 | 54.2 | 38.7 | 20.7 | 0.223 |
| 11k | 66.1 | 65.6 | 62.9 | 53.8 | 39.5 | 22.7 | 0.224 |
11l![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) b b |
45.1 | 44.2 | 42.1 | 49.6 | 40.1 | 23.9 | >10 |
| 11m | 68.9 | 66.5 | 63.8 | 52.8 | 37.0 | 18.6 | 0.247 |
| 11n | 74.5 | 73.3 | 70.0 | 59.2 | 41.0 | 0.172 | |
| 11o | 68.4 | 65.9 | 61.6 | 47.0 | 29.0 | 14.1 | 0.385 |
| 11p | 68.1 | 65.2 | 60.3 | 46.8 | 29.3 | 14.8 | 0.399 |
| 11q | 62.8 | 66.2 | 60.3 | 48.2 | 40.3 | 16.7 | 0.359 |
| 11r | 57.3 | 52.1 | 46.9 | 39.5 | 28.7 | 14.0 | 1.925 |
| 12a | 69.2 | 67.5 | 65.2 | 56.6 | 41.8 | 23.9 | 0.184 |
| 12b | 71.5 | 68.8 | 65.1 | 52.5 | 37.3 | 20.7 | 0.250 |
12c![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) c c |
57.0 | 55.2 | 52.0 | 42.2 | 28.8 | 17.8 | 0.782 |
12d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) d d |
57.6 | 55.1 | 53.0 | 46.1 | 34.9 | 18.9 | 0.529 |
| 12e | 69.8 | 69.1 | 67.9 | 62.8 | 54.4 | 40.2 | 0.069 |
| 12f | 66.8 | 65.0 | 63.4 | 59.6 | 48.4 | 32.0 | 0.117 |
| 12g | 67.8 | 65.3 | 64.2 | 59.8 | 49.7 | 32.4 | 0.103 |
| 12h | 69.5 | 68.8 | 67.7 | 64.6 | 56.7 | 39.6 | 0.062 |
| 12i | 68.5 | 68.6 | 67.1 | 63.5 | 54.8 | 38.9 | 0.070 |
| 12j | 68.9 | 69.6 | 68.8 | 66.7 | 61.3 | 47.4 | 0.038 |
12k![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) e e |
72.3 | 70.6 | 70.3 | 68.3 | 62.9 | 49.3 | 0.032 |
12l![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) f f |
70.1 | 66.4 | 67.4 | 63.9 | 60.4 | 48.9 | 0.034 |
12m![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) g g |
72.2 | 70.7 | 70.0 | 67.0 | 60.9 | 46.0 | 0.041 |
| 12n | 71.6 | 71.0 | 70.4 | 66.9 | 58.6 | 42.3 | 0.053 |
| 12o | 70.2 | 69.6 | 68.6 | 66.6 | 61.0 | 46.3 | 0.041 |
| 12p | 69.0 | 67.1 | 65.7 | 60.6 | 50.2 | 34.3 | 0.098 |
12q![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) h h |
67.6 | 66.0 | 65.4 | 62.9 | 57.0 | 42.9 | 0.055 |
| 12r | 69.8 | 68.2 | 68.7 | 64.4 | 57.5 | 39.6 | 0.060 |
| 12s | 69.1 | 69.7 | 69.0 | 67.7 | 64.0 | 46.9 | 0.037 |
Allo![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) i i |
38.2 | 19.9 | 9.9 | 4.6 | 3.2 | 24.3 | |
Conclusion
Thus, this simple and general methodology provided a facile and convenient route to the preparation of 3-substituted 7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (12), which were obtained by oxidative cyclisation of the corresponding 4-aldehyde hydrazones of 1H-pyrazolo[3,4-d
)-ones (12), which were obtained by oxidative cyclisation of the corresponding 4-aldehyde hydrazones of 1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidines (3 and 11) with 70% nitric acid as the key step, as a new class of potential xanthine oxidase inhibitors. Their inhibitory activities against bovine milk xanthine oxidase in vitro were investigated, and some 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d
]pyrimidines (3 and 11) with 70% nitric acid as the key step, as a new class of potential xanthine oxidase inhibitors. Their inhibitory activities against bovine milk xanthine oxidase in vitro were investigated, and some 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (11) exhibited from several times to several hundred times more potent activities than allopurinol. In addition, the tricyclic compounds, 3-aryl-7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H
)-ones (11) exhibited from several times to several hundred times more potent activities than allopurinol. In addition, the tricyclic compounds, 3-aryl-7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-ones (12) showed potent inhibitory activities, being ca. three orders of magnitude more active than allopurinol. They did not show any appreciable inhibition against the proliferation of T-cell acute lymphoblastic leukemia (CCRF-HSB-2) however.†
Biological testing of the compounds in vivo is now ongoing and the results will be reported later.
)-ones (12) showed potent inhibitory activities, being ca. three orders of magnitude more active than allopurinol. They did not show any appreciable inhibition against the proliferation of T-cell acute lymphoblastic leukemia (CCRF-HSB-2) however.†
Biological testing of the compounds in vivo is now ongoing and the results will be reported later.
Experimental
General
Mps were obtained on a Yanagimoto micro melting point apparatus and were uncorrected. Microanalyses were measured by a Yanaco CHN Corder MT-5 apparatus. Mass spectra were recorded at 70 eV ionizing voltage with FAB ionization using a VG-70SE spectrometer and 3-nitrobenzyl alcohol or glycerol as a matrix. IR spectra were recorded using a JASCO FT/IR-200 spectrophotometer as Nujol mulls. 1H NMR spectra were obtained using Hitachi FT-NMR R-1500 (60 MHz) and Varian VXR 200 MHz spectrometers. In all cases, chemical shifts are in ppm relative to SiMe4 as internal standard and J values are given in Hz. All reagents were of commercial quality from freshly opened containers and were used without further purification. Organic solvents were dried by standard methods and distilled before use. Reaction progress was monitored by analytical thin layer chromatography (TLC) on pre-coated glass plates (silica gel 70 FM Plate-Wako) using the following solvent systems: (A) AcOEt–EtOH (4∶1 v/v), (B) EtOH, (C) MeOH and others cited in the Tables. The products were visualized by UV light. Column chromatography was run on Daisogel IR-60 (63/210 μM, Daiso Co.). The reaction temperatures are indicated as the temperature of oil bath.6-Chloro-4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidine 2
]pyrimidine 2
To a stirring solution of 2,4,6-trichloropyrimidine-5-carbaldehyde 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 22 (3.0 g, 14.2 mmol) in 2-methoxyethanol (20 cm3) at 0 °C was added a solution of anhydrous hydrazine (1.82 g, 56.8 mmol) diluted with 2-methoxyethanol (18 cm3) in limited amounts for 30 min. After the reaction was complete, the precipitated crystals were collected by filtration and washed with water and EtOH to afford the pyrazolopyrimidine2 (2.06 g, 79%) as pale yellow powdery crystals, mp > 300 °C; Rf (A) 0.64; νmax/cm−1 3350 and 3260 (NH2), 3160 and 3100 (NH) and δmax/cm−1 1660 (NH2); δH [60 MHz; (CD3)2SO] 4.20 (2 H, br, NH2), 8.50 (1 H, s, 3-H), 9.45 (1 H, br, 4-NH) and 13.40 (1 H, br, 1-NH); m/z (FAB, 3-nitrobenzyl alcohol matrix) 185 (MH+) and 187 (MH+ + 2). The product 2 was obtained as a single compound and was used for the following reactions without further purification because it was difficult to purify since it was insoluble in usual solvents.
22 (3.0 g, 14.2 mmol) in 2-methoxyethanol (20 cm3) at 0 °C was added a solution of anhydrous hydrazine (1.82 g, 56.8 mmol) diluted with 2-methoxyethanol (18 cm3) in limited amounts for 30 min. After the reaction was complete, the precipitated crystals were collected by filtration and washed with water and EtOH to afford the pyrazolopyrimidine2 (2.06 g, 79%) as pale yellow powdery crystals, mp > 300 °C; Rf (A) 0.64; νmax/cm−1 3350 and 3260 (NH2), 3160 and 3100 (NH) and δmax/cm−1 1660 (NH2); δH [60 MHz; (CD3)2SO] 4.20 (2 H, br, NH2), 8.50 (1 H, s, 3-H), 9.45 (1 H, br, 4-NH) and 13.40 (1 H, br, 1-NH); m/z (FAB, 3-nitrobenzyl alcohol matrix) 185 (MH+) and 187 (MH+ + 2). The product 2 was obtained as a single compound and was used for the following reactions without further purification because it was difficult to purify since it was insoluble in usual solvents.
4-Alkylidenehydrazino- and 4-arylmethylidenehydrazino-6-chloro-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidines 3a–g; General procedure
]pyrimidines 3a–g; General procedure
A mixture of the hydrazinopyrazolopyrimidine 2 (1.0 g, 5.42 mmol) and an appropriate alkyl aldehyde or aryl aldehyde (6.50 mmol) in DMF (50 cm3) was stirred at room temperature for 10 hours. After the reaction was complete, the solution was evaporated under reduced pressure and the residue was triturated with EtOH or AcOEt to give crystals, which were collected by filtration and recrystallized from an appropriate solvent to afford the corresponding hydrazones3a–g as shown in Tables 1 and 2.
1H-Pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidine-4,6(5H,7H
]pyrimidine-4,6(5H,7H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-dione 4 (oxypurinol)
)-dione 4 (oxypurinol)
(1) A mixture of the hydrazino derivative 2 (0.20 g, 1.08 mmol) with concentrated hydrochloric acid (10 cm3) was heated under reflux for 1 hour. After the reaction was complete, the solution was treated with activated charcoal and evaporated under reduced pressure; the residue was recrystallized from water to afford oxypurinol [95 mg, 58%; mp > 300 °C; Rf (A) 0.48; νmax/cm−1 3180, 3150 and 3120 (NH) and 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O)], which was identical with an authentic sample.23
O)], which was identical with an authentic sample.23
(2) The hydrazone 3b (0.50 g, 1.83 mmol) in 10% aqueous HCl (50 cm3) was heated under reflux for 3 hours. After the reaction was complete, the solution was treated with activated charcoal and cooled to afford a deposit, which was collected by filtration. The filtrate was evaporated under reduced pressure and the residue was recrystallized from water to get the second crop. The product was identical with oxypurinol (150 mg, 54%).
4-Hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-one 6
)-one 6
To a mixture of hydrazine monohydrate (5.0 g, 99.9 mmol) and ethanol (5 cm3) was added 4,5-dihydro-4-thioxo-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 5
)-one 5![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 23 (1.0 g, 5.95 mmol) and the mixture was heated under reflux for 10 min. After the reaction was complete, the precipitated crystals were collected by filtration and washed with water and EtOH to afford the hydrazino derivative6 (0.70 g, 71%) as colourless powdery crystals, mp > 300 °C; Rf (B) 0.28; νmax/cm−1 3360 and 3310 (NH2), 3200, 3150 and 3100 (NH), 1710 (C
23 (1.0 g, 5.95 mmol) and the mixture was heated under reflux for 10 min. After the reaction was complete, the precipitated crystals were collected by filtration and washed with water and EtOH to afford the hydrazino derivative6 (0.70 g, 71%) as colourless powdery crystals, mp > 300 °C; Rf (B) 0.28; νmax/cm−1 3360 and 3310 (NH2), 3200, 3150 and 3100 (NH), 1710 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) and δmax/cm−1 1670 (NH2); δH [60 MHz; CF3CO2D] 8.72 (1 H, s, 3-H); m/z (FAB, 3-nitrobenzyl alcohol matrix) 167 (MH+). The product 6 was obtained as a single compound and was used for the following reactions without further purification because it was difficult to purify since it was insoluble in usual solvents.
O) and δmax/cm−1 1670 (NH2); δH [60 MHz; CF3CO2D] 8.72 (1 H, s, 3-H); m/z (FAB, 3-nitrobenzyl alcohol matrix) 167 (MH+). The product 6 was obtained as a single compound and was used for the following reactions without further purification because it was difficult to purify since it was insoluble in usual solvents.
4,6-Dihydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidine 7
]pyrimidine 7
(1) To a stirring solution of 2,4,6-trichloropyrimidine-5-carbaldehyde 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 22 (3.0 g, 14.2 mmol) in 2-methoxyethanol (20 cm3) at 0 °C was added anhydrous hydrazine (9.1 g, 283.9 mmol) dropwise. Then, the stirred mixture was heated at 100 °C for 5 hours. After the reaction was complete, the precipitated crystals were collected by filtration, washed with water and EtOH and recrystallized from water to afford the dihydrazino derivative7 (1.94 g, 76%) as colourless powdery crystals, mp > 300 °C (Found: C, 33.1; H, 4.55; N, 61.5. C5H8N8·1/5 H2O requires C, 32.7; H, 4.6; N, 61.0%); Rf (C) 0.20; νmax/cm−1 3335, 3270 and 3230 (NH2), 3180, 3120 and 3110 (NH) and δmax/cm−1 1650 and 1640 (NH2); δH [60 MHz; CF3CO2D] 8.81 (1H, s, 3-H); m/z (FAB, 3-nitrobenzyl alcohol matrix) 181 (MH+).
22 (3.0 g, 14.2 mmol) in 2-methoxyethanol (20 cm3) at 0 °C was added anhydrous hydrazine (9.1 g, 283.9 mmol) dropwise. Then, the stirred mixture was heated at 100 °C for 5 hours. After the reaction was complete, the precipitated crystals were collected by filtration, washed with water and EtOH and recrystallized from water to afford the dihydrazino derivative7 (1.94 g, 76%) as colourless powdery crystals, mp > 300 °C (Found: C, 33.1; H, 4.55; N, 61.5. C5H8N8·1/5 H2O requires C, 32.7; H, 4.6; N, 61.0%); Rf (C) 0.20; νmax/cm−1 3335, 3270 and 3230 (NH2), 3180, 3120 and 3110 (NH) and δmax/cm−1 1650 and 1640 (NH2); δH [60 MHz; CF3CO2D] 8.81 (1H, s, 3-H); m/z (FAB, 3-nitrobenzyl alcohol matrix) 181 (MH+).
(2) To a stirring solution of 80% aqueous hydrazine hydrate (20 cm3, 320 mmol) was added 6-chloro-4-hydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidine 2 (2.0 g, 10.8 mmol) and the mixture was heated at 80–90 °C for 4 hours. After the same work-up as noted above, recrystallization of the crude crystals from water gave the dihydrazino derivative7 (1.40 g, 72%).
]pyrimidine 2 (2.0 g, 10.8 mmol) and the mixture was heated at 80–90 °C for 4 hours. After the same work-up as noted above, recrystallization of the crude crystals from water gave the dihydrazino derivative7 (1.40 g, 72%).
4,6-Bis(arylmethylidenehydrazino)-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidines 8b–d, f. General procedure
]pyrimidines 8b–d, f. General procedure
A mixture of the dihydrazino derivative 7 (0.60 g, 3.33 mmol) and an appropriate aldehyde (9.99 mmol) in DMF (20 cm3) was stirred at room temperature for 10 hours. After the reaction was complete, the solution was evaporated under reduced pressure and the residue was triturated with EtOH to give crystals, which were collected by filtration and recrystallized from a mixture of EtOH and DMF to afford the corresponding bishydrazones8b–d, f as shown in Tables 1 and 2.
4-Carbamoylhydrazino-6-chloro-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidine 9
]pyrimidine 9
A mixture of the hydrazinopyrazolopyrimidine 2 (0.5 g, 2.71 mmol) and urea (0.65 g, 10.8 mmol) in 2-ethoxyethanol (25 cm3) was heated under reflux for 5 hours. After the reaction was complete, the solution was evaporated under reduced pressure to afford a solid. The solid was collected by filtration, washed with water and recrystallized from water to afford the carbamoylhydrazino derivative9 (0.37 g, 60%) as colourless powdery crystals, mp > 300 °C (Found: C, 31.1; H, 2.9; N, 43.0. C6H6ClN7O·1/7 H2O requires C, 31.3; H, 2.75; N, 42.6%); Rf (B) 0.70; νmax/cm−1 3410 and 3360 (NH2), 3200, 3100 and 3040 (NH), 1675 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) and δmax/cm−1 1675 (NH2); δH [200 MHz; (CD3)2SO] 6.25 (2 H, br, NH2), 7.94 (1 H, s, 3-H), 8.66 and 10.00 (each 1 H, each br s, 2 × NH) and 13.67 (1 H, br s, 1-NH); m/z (FAB, glycerol matrix) 228 (MH+) and 230 (MH+ + 2).
O) and δmax/cm−1 1675 (NH2); δH [200 MHz; (CD3)2SO] 6.25 (2 H, br, NH2), 7.94 (1 H, s, 3-H), 8.66 and 10.00 (each 1 H, each br s, 2 × NH) and 13.67 (1 H, br s, 1-NH); m/z (FAB, glycerol matrix) 228 (MH+) and 230 (MH+ + 2).
3-Amino-7H-pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-one 10
)-one 10
(1) The reaction mixture in the same reaction and under the same conditions as in the above preparation for 9 was heated under reflux for 36 hours. After the same work-up as noted above, recrystallization of the crude crystals from water gave the pyrazolotriazolopyrimidine 10 (0.15 g, 29%) as colourless powdery crystals, mp > 300 °C (Found: C, 37.1; H, 3.1; N, 49.9. C6H5N7O·1/4 H2O requires C, 36.8; H, 2.8; N, 50.1%); Rf (A) 0.57; νmax/cm−1 3370 and 3260 (NH2), 3180 and 3100 (NH), 1720 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) and δmax/cm−1 1685 (NH2); δH [200 MHz; (CD3)2SO] 7.83 (1 H, s, 9-H), 7.94 (2 H, br s, NH2), 12.27 (1 H, br s, 6-NH) and 13.10 (1 H, br s, 7-NH); m/z (FAB, glycerol matrix) 192 (MH+).
O) and δmax/cm−1 1685 (NH2); δH [200 MHz; (CD3)2SO] 7.83 (1 H, s, 9-H), 7.94 (2 H, br s, NH2), 12.27 (1 H, br s, 6-NH) and 13.10 (1 H, br s, 7-NH); m/z (FAB, glycerol matrix) 192 (MH+).
(2) A mixture of the pyrazolopyrimidine 9 (0.2 g, 0.88 mmol) and urea (0.16 g, 2.66 mmol) in 2-ethoxyethanol (10 cm3) was heated under reflux for 10 hours. After the same work-up as noted above, recrystallization of the crude crystals from water gave the pyrazolotriazolopyrimidine10 (30 mg, 18%).
4-Alkylidenehydrazino- and 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-ones 11b, e–r. General procedure
)-ones 11b, e–r. General procedure
A mixture of the hydrazinopyrazolopyrimidine 6 (1.0 g, 6.02 mmol) and an appropriate alkyl aldehyde or aryl aldehyde (9.03 mmol) in DMF (50 cm3) was stirred at room temperature or 40 °C for 10 hours. After the reaction was complete, the solution was evaporated under reduced pressure and the residue was triturated with EtOH or AcOEt to give crystals, which were collected by filtration and recrystallized from an appropriate solvent to afford the corresponding hydrazones11b, e–r as shown in Tables 3 and 4.
7H-Pyrazolo[4,3-e]-1,2,4-triazolo[4,3-c]pyrimidin-5(6H![[hair space]](https://www.rsc.org/images/entities/h3_char_200a.gif) )-one 12a and its 3-substituted derivatives 12b–s. General procedure
)-one 12a and its 3-substituted derivatives 12b–s. General procedure
(1) Method A: A mixture of an appropriate 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 11b, e–r (2.0 mmol) with 70% nitric acid (0.22 cm3, 2.4 mmol) in DMF (30–50 cm3) was heated at 100 °C for 1–9 hours. After the reaction was complete, the precipitated crystals were collected by filtration and combined with further material obtained by concentration of the filtrate under reduced pressure. The crystals were recrystallized from an appropriate solvent to afford the corresponding pyrazolotriazolopyrimidines12b, e–p, r, s as shown in Tables 5 and 6.
)-one 11b, e–r (2.0 mmol) with 70% nitric acid (0.22 cm3, 2.4 mmol) in DMF (30–50 cm3) was heated at 100 °C for 1–9 hours. After the reaction was complete, the precipitated crystals were collected by filtration and combined with further material obtained by concentration of the filtrate under reduced pressure. The crystals were recrystallized from an appropriate solvent to afford the corresponding pyrazolotriazolopyrimidines12b, e–p, r, s as shown in Tables 5 and 6.
(2) Method B: A mixture of an appropriate 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidin-6(7H
]pyrimidin-6(7H![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) )-one 11e, g, h, k, m–o, q (2.0 mmol) with DEAD (0.35 g, 2.0 mmol) in DMF (50 cm3) was heated under reflux. After heating for several hours, further DEAD (2.0 mmol amounts; total 3–7 equiv.) was added to the heated solution at hourly intervals until the hydrazone 11 disappeared. After the reaction was complete, the solution was evaporated under reduced pressure to leave a solid, which was purified by column chromatography on silica gel using AcOEt as eluent and recrystallized from an appropriate solvent to give the corresponding pyrazolotriazolopyrimidines12e, g, h, k, m–o, q as shown in Tables 5 and 6.
)-one 11e, g, h, k, m–o, q (2.0 mmol) with DEAD (0.35 g, 2.0 mmol) in DMF (50 cm3) was heated under reflux. After heating for several hours, further DEAD (2.0 mmol amounts; total 3–7 equiv.) was added to the heated solution at hourly intervals until the hydrazone 11 disappeared. After the reaction was complete, the solution was evaporated under reduced pressure to leave a solid, which was purified by column chromatography on silica gel using AcOEt as eluent and recrystallized from an appropriate solvent to give the corresponding pyrazolotriazolopyrimidines12e, g, h, k, m–o, q as shown in Tables 5 and 6.
(3) Method C: A mixture of the hydrazinopyrazolopyrimidine 6 (0.60 g, 3.6 mmol) with an appropriate triethyl orthoester (18.0 mmol) in trifluoroacetic acid (9 cm3) was stirred at room temperature for 1 hour. After the reaction was complete, the precipitated crystals were collected by filtration and recrystallized from an appropriate solvent to afford the corresponding pyrazolotriazolopyrimidines12a, b as shown in Tables 5 and 6.
(4) Method D: A mixture of the hydrazinopyrazolopyrimidine 6 (0.60 g, 3.6 mmol) with an appropriate triethyl or trimethyl orthoester (10.8 mmol) in DMF (30–40 cm3) was heated at 100 °C for 1 hour. After the reaction was complete, the solution was evaporated under reduced pressure and the residue was triturated with EtOH or AcOEt to give crystals, which were collected by filtration and recrystallized from an appropriate solvent to afford the corresponding pyrazolotriazolopyrimidines12a–d as shown in Tables 5 and 6.
(5) Method E: A mixture of an appropriate 4-alkylidenehydrazino- or 4-arylmethylidenehydrazino-6-chloro-1H-pyrazolo[3,4-d![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ]pyrimidine 3a–g (2.0 mmol) with 70% nitric acid (0.9 cm3, 10.0 mmol) in DMF (30–50 cm3) was heated at 100 °C for 1–5 hours. After the reaction was complete, the precipitated crystals were collected by filtration and further crystals were obtained by concentration of the filtrate under reduced pressure. The combined crystals were recrystallized from an appropriate solvent to afford the corresponding pyrazolotriazolopyrimidines12e, g, h, k, m, n, r as shown in Tables 5 and 6.
]pyrimidine 3a–g (2.0 mmol) with 70% nitric acid (0.9 cm3, 10.0 mmol) in DMF (30–50 cm3) was heated at 100 °C for 1–5 hours. After the reaction was complete, the precipitated crystals were collected by filtration and further crystals were obtained by concentration of the filtrate under reduced pressure. The combined crystals were recrystallized from an appropriate solvent to afford the corresponding pyrazolotriazolopyrimidines12e, g, h, k, m, n, r as shown in Tables 5 and 6.
Xanthine oxidase assay
All test compounds and allopurinol were dissolved in dimethyl sulfoxide (DMSO) and diluted with 50 mM sodium phosphate buffer (pH 7.4) for in vitro experiments. The final concentration of DMSO in the reaction solution was 0.1%.Bovine milk xanthine oxidase (XO) (10 mU ml−1) was incubated with 100 μM xanthine in the presence and absence of the test compound (0.003–10 μM) at 25 °C for 15 min. Uric acid formation was determined by absorbance at 292 nm using a Hitachi 228-A spectrophotometer, and the inhibition rate (%) for the formation of uric acid and IC50 values of the test compounds were determined. The inhibition rate (I) of the test compound at each concentration was calculated by eqn. (1), where T is the optical density of a solution of xanthine and XO, D is the optical density of a solution of test compound, xanthine and XO and DB is the optical density of a solution of test compound and XO.
| I (%) = 100 − [(D − DB)/T] × 100 | (1) |
The inhibitory activity of allopurinol against bovine milk xanthine oxidase was also examined as a positive control. Each experiment was repeated at least twice at different concentrations (0.003–10 μM). The values of IC50, i.e. the μM concentration of inhibitor necessary for 50% inhibition, were determined from the dose–response curve from the relation of the logarithmic concentration (μM) and the inhibition (%).
Acknowledgements
We are grateful to the SC-NMR Laboratory of Okayama University for 200 MHz proton NMR experiments. This work was supported in part by a Grant-in-Aid for Scientific Research (C) (No. 09680570) from the Japan Society for the Promotion of Science.References
- Part 2 T. Nagamatsu, H. Yamasaki, T. Fujita, K. Endo and H. Machida, J. Chem. Soc., Perkin Trans. 1, 1999, 3117 RSC.
- R. W. Rundles, J. B. Wyngaarden, G. H. Hitchings, G. B. Elion and H. R. Silberman, Trans. Assoc. Am. Physicians, 1963, 76, 126 Search PubMed.
- T. F. Yü and A. B. Gutman, Am. J. Med., 1964, 37, 885 CrossRef CAS.
- J. R. Klinenberg, S. E. Goldfinger and J. E. Seegmiller, Ann. Intern. Med., 1965, 62, 639 Search PubMed.
- G. B. Elion, Ann. Rheumat. Dis., 1966, 25, 608 Search PubMed.
- G. B. Elion, S. Callahan, H. Nathan, S. Bieber, R. W. Rundles and G. H. Hitchings, Biochem. Pharmacol., 1963, 12, 85 CrossRef CAS.
- J. L. Young, R. B. Boswell and A. S. Nies, Arch. Intern Med., 1974, 134, 553 Search PubMed.
- K. R. Hande, R. M. Noone and W. J. Stone, Am. J. Med., 1984, 76, 47 CrossRef CAS.
- D. E. Duggan, R. M. Noll, J. E. Baer, F. C. Novello and J. J. Baldwin, J. Med. Chem., 1975, 18, 900 CrossRef CAS.
- R. L. Wortmann, A. S. Ridolfo, R. W. Lightfoot Jr. and I. H. Fox, J. Rheumatol., 1985, 12, 540 Search PubMed.
- A. Bindoli, M. Valente and L. Cavallini, Pharmacol. Res. Commun., 1985, 17, 831 Search PubMed.
- T. Spector, W. W. Hall, D. J. Porter, C. U. Lambe, D. J. Nelson and T. A. Krenitsky, Biochem. Pharmacol., 1989, 38, 4315 CrossRef CAS.
- S. Sato , K. Tatsumi and T. Takahashi , Purine and Pyrimidine Metabolism in Man VII, Part A: Chemotherapy, ATP Depletion and Gout, eds. R. A. Harkness, G. B. Elion and N. Zöllner, Plenum Press, New York, 1991, p. 135. Search PubMed.
- Y. Osada, M. Tsuchimoto, H. Fukushima, K. Takahashi, S. Kondo, M. Hasegawa and K. Komoriya, Eur. J. Pharmacol., 1993, 241, 183 CrossRef CAS.
- G. Biagi, I. Giorgi, O. Livi, V. Scartoni, I. Tonetti and L. Costantino, Farmaco, 1995, 50, 257 CAS.
- T. Nagamatsu , Y. Watanabe , K. Endo and M. Imaizumi , PCT Int. Appl. WO 96 26,208/1996(Chem. Abstr., 1996, 125, 247848j). Search PubMed.
- T. Nagamatsu , T. Abiru , Y. Watanabe and K. Endo , Jpn. Kokai Tokkyo Koho JP 07,242,694/1995(Chem. Abstr., 1996, 124, 117896s). Search PubMed.
- T. Nagamatsu and T. Fujita, Chem. Commun., 1999, 1461 RSC.
- G. A. Bhat and L. B. Townsend, J. Chem. Soc., Perkin Trans. 1, 1981, 2387 RSC.
- F. Gatta, M. Luciani and G. Palazzo, J. Heterocycl. Chem., 1989, 26, 613 Search PubMed.
- U. D. Treuner and H. Breuer , USP 4 053 474, 1977(Chem. Abstr., 1977, 88, 37826s) Search PubMed; USP 4 124 764, 1978 (Chem. Abstr., 1978, 90, 87508b) Search PubMed; Ger. Offen. 2 838 029, 1979 (Chem. Abstr., 1979, 91, 39492r). Search PubMed.
- F. Yoneda, Y. Sakuma, S. Mizumoto and R. Ito, J. Chem. Soc., Perkin Trans. 1, 1976, 1805 RSC.
- R. K. Robins, J. Am. Chem. Soc., 1956, 78, 784 CrossRef CAS.
Footnote |
| † We have found that some derivatives exhibited poor inhibitory activities against the proliferation of T-cell acute lymphoblastic leukemia (CCRF-HSB-2): the IC50 for 11j, 11 μM; for 11o, 45 μM; for 12i, 25 μM; for 12j, 14 μM; for 12k, 35 μM; for 12l, 23 μM; for 12n, 36 μM; for 12q, 34 μM; for 12r, 36 μM; for 12s, 35 μM and for arabinosylcytosine, 0.061 μM. |
| This journal is © The Royal Society of Chemistry 2000 |

