Condensation of organic vapours within nanoporous calixarene thin films †
Alexey V. Nabok, Aseel K. Hassan and Asim K. Ray*
Electronic Research Group, School of Engineering, Sheffield Hallam University, Pond Street, Sheffield, UK S1 1WB
First published on UnassignedUnassigned22nd December 1999
Abstract
Adsorption of vapours of benzene, toluene, p-xylene, aniline, hexane and chloroform in LB films of two novel calix[4]resorcinarene derivatives was studied in situ using quartz-crystal microbalance (QCM), ellipsometry and surface plasmon resonance (SPR) techniques. Isotherms of adsorption obtained by both QCM and SPR show that the adsorption ability depends on the condensed vapour pressures of the adsorbates rather than on a structural coincidence between host cavities and guest molecules. The results were interpreted in terms of capillary condensation of organic vapours in the nanoporous matrix of calixarene LB films accompanied by film swelling. Ellipsometric measurements show changes of both the thickness and refractive index of the LB films caused by adsorption, and thus confirm condensation and further accumulation of liquid adsorbate within the film matrix. Unusual adsorption kinetics were observed only when the vapour injection technique was used and their occurrence is believed to be caused by initial vapour condensation on the film surface. This can be eliminated by measurements under constant vapour flow.
Introduction
Atmospheric detection of odorant vapours of organic compounds is one of the most important problems of environment monitoring. A number of vapour sensors 1–9 and sensor arrays 10–13 have been developed during the last few years. The majority of these sensors are based on the use of different polymer coatings 1,3,5,6,11,12 and employ several transduction techniques, such as, using a mass sensitive quartz crystal microbalance (QCM) 1,2,6,7,13 and surface acoustic wave (SAW) 3,8 devices, ChemFETs (chemical field effect transistors), 9 conductivity 1,4,5,12 and interdigitated capacitance 10 measurements. It was shown recently that some novel cavitand compounds such as crown ethers 8 and calixarenes 14,15 can form inclusion complexes with some organic guest molecules, and this effect can be used for the development of sensors for organic vapours. 16–20Several attempts to study the adsorption of organic vapours within thin calixarene films formed with different techniques, including LB film deposition, spin coating and self-assembly, have been made. 16–22 In recent publications 21,22 we have shown that the vapours of benzene and toluene, as well as some hydrocarbons (hexane), can be adsorbed at calixarene Langmuir–Blodgett (LB) films. This adsorption process is very fast, and full recovery of the film has been observed after flushing with clean air. It has to be pointed out, however, that the detected vapours were of a high concentration (a few percent in volume) and the adsorption was not selective since all vapours studied yielded a similar response. These effects are attributed to weak and non-specific interactions between guest molecules and the calixarene LB film. It was also shown that the adsorption of organic vapours occurs in the whole bulk of the LB films, and that the number of adsorbed molecules is much higher than the number of calixarene molecules. 21 The proposed mechanism of adsorption included swelling of the film and even condensation of adsorbate within the film. The swelling of the film has been confirmed directly by ellipsometry and surface plasmon resonance (SPR) measurements. 21,22 but the mechanism of adsorption is still unclear.
Different calix[4]resorcinarene derivatives have been deposited by self-assembly and spin-coating techniques onto QCM and SAW devices, and their response to various organic vapours has been studied. 23 Although a few particular calixarene–vapour combinations have shown a high selective host–guest type molecular recognition, the majority of calixarene compounds have resulted in a more or less similar response to different organic vapours. It seems that the cavitand nature of sensing molecules is not necessary for this mechanism of adsorption, since a similar effect has been observed in phthalocyanine LB films in response to their exposure to toluene vapours. 24
The presence of alkyl chains is important for this type of complexation. All the organic guests investigated are solvents in the liquid phase, and they can therefore interact with the alkyl chains of amphiphilic calixarene derivatives. This has been proved by NMR spectral measurements of the complexation of toluene with phosphorylated calix[4]resorcinarenes. 25 In contrast, thin films of unsubstituted calix[ n]arenes, produced by vacuum evaporation, show a permanent binding of benzene derivatives, possibly, due to the formation of inclusion complexes. 26
In our previous study of the adsorption kinetics using ellipsometry and SPR, an initial sharp response on vapour injection followed by a decay and stabilisation has been recorded. 21,22 It was suggested that such unusual adsorption kinetics could be attributed either to formation of liquid state adsorbate on the film surface or to some possible experimental artefacts.
Taking into consideration all known experimental facts, we can assume a more complex adsorption mechanism. This includes, in addition to the conventional host–guest binding within calixarene cavities, the interaction of guest molecules with alkyl chains, their penetration into the film bulk through pores, as well as their further condensation inside the film in a liquid state. In order to prove this mechanism, the adsorption of different organic molecules such as aromatic compounds (benzene, toluene, p-xylene, etc.), hydrocarbons (hexane) and chloro-hydrocarbons (chloroform), in LB films of several calixarene derivatives was studied using a number of methods including QCM, ellipsometry, and SPR.
Two amphiphilic calix[4]resorcinarene derivatives were used in the present study. These are phosphorylated and azobenzene substituted compounds, referred to throughout the text as P-C[4]RA and Azo-C[4]RA, respectively. The chemical structures of these compounds are shown in Fig. 1. The synthesis of these calixarene derivatives has been described previously. 25,27 P-C[4]RA molecule has a boat-like conformation, 25 while Azo-C[4]RA forms a cone with the cavity extended by the presence of four azobenzene groups. 27 The difference in the shape of these two compounds may cause variation in their adsorption properties.
![Chemical structures of (a) P-C[4]RA and (b) Azo-C[4]RA derivatives.](/image/article/2000/JM/a903502h/a903502h-f1.gif) | ||
| Fig. 1 Chemical structures of (a) P-C[4]RA and (b) Azo-C[4]RA derivatives. | ||
Film preparation and experimental details
Langmuir–Blodgett films of the compounds mentioned above were produced using a NIMA 622 LB trough equipped with a Wilhelmy balance system. Floating layers of C[4]RA derivatives were formed onto the surface of Milli-Q water by spreading a 0.5 mg ml −1 solution of these in chloroform. Y-type LB films were transferred onto hydrophobic substrates at a constant surface pressure of about 20 mN m −1 and a dipping speed of 10 mm min −1 for both down- and up-strokes. To make the substrates hydrophobic, the silicon wafers and the quartz crystals used were exposed previously to hexamethyldisilazane vapours as described earlier. 21,22 Glass slides with freshly evaporated 45 nm thick gold layers, having a good hydrophobic surface, were used as prepared.The QCM set-up, consisting of an electrical oscillating circuit and a gas chamber, was made in-house. Certain concentrations of odorant vapours, in particular toluene and hexane, were prepared within the chamber by injecting the required amount of liquid toluene and hexane. The frequency of the quartz oscillator (which was in the range 10–11 MHz) was monitored using a frequency-counter, and readings were taken twice before an exposure and under steady state conditions, when evaporation of the liquid droplet within the chamber was complete and the equilibrium vapour concentration had been formed.
Ellipsometric measurements were carried out using a zero-type LEF 3M instrument equipped with HeNe (632.8 nm) laser source and a specially designed gas cell described previously. 21 Both polariser and analyser were fixed at positions (angles) of P0 and A0, respectively, providing the minimum of the output light intensity. Initial values of the thickness ( d) and refractive index ( n) of the film were calculated from P0 and A0. In order to follow the adsorption kinetics, the photodetector output signal was monitored on a chart-recorder during the injection of vapour into the gas cell. When steady state adsorption was reached, a new set of readings ( A1, P1) were taken and new values of d and n were calculated. Certain concentrations of vapour were achieved by diluting saturated vapour with air.
SPR measurements were carried out using the Kretschmann type θ–2 θ rotated stage experimental set-up described previously. 22 The samples were brought into optical contact with the equilateral prism using an index-matching liquid (ethyl salicylate from Aldrich). A p-polarised HeNe laser beam ( λ = 632.8 nm) was used for excitation of surface plasmons. A gas cell, sealed at the sample through a rubber O-ring, was used to study vapour adsorption in the calixarene LB films. Kinetic SPR measurements of the intensity of reflected light at the fixed angle θ*, chosen near the SPR minimum on the left side of the SPR curve, were performed in situ during exposure to organic vapour.
In order to study the adsorption kinetics in detail, three methods of vapour preparation were employed in the SPR measurements: (i) injection of vapours at a certain concentration, prepared by dilution of the saturated vapours, into the gas cell; (ii) injection of a small amount of liquid solvent into the gas cell and the slow formation of the saturated vapour during its evaporation; and (iii) measurements in a constant vapour flow. A Standard Vapour Generator (A.I.D. model 350) was used in the latter case. The vapour concentration was calculated for a particular solvent at a certain temperature, knowing the geometrical dimensions of the diffusion sample tube used. 28
Results and discussion
QCM study of adsorption of vapours
The oscillating frequency of quartz crystals coated with calixarene LB films was monitored during their exposure to toluene and hexane vapours. Changes in the mass of quartz crystals (Δ m/g cm −2), caused by adsorption of the vapours, were calculated from the frequency shift (Δ f/Hz) of QCM as in eqn. (1). 21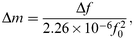 | (1) |
where f0 (10–11 MHz) is the nominal frequency of quartz crystals. This formula was derived from the Sauerbrey equation, 29 based upon an ideal mass approach and valid for thin films causing frequency changes not more than 1% of its nominal value. The concentration of adsorbed guest molecules Nads can be calculated from eqn. (2).
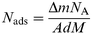 | (2) |
where NA is Avogadro's number, A is the area of the quartz crystal, d is the thickness of LB coating and M = 92 and 84, the molecular weights of toluene and hexane, respectively. Knowing the concentration of calixarene molecules in the LB films, one can calculate the number of adsorbed guest molecules per calixarene moiety.
Fig. 2 shows typical results obtained by QCM measurements on quartz crystals coated with LB films (30 layers) of P-C[4]RA when exposed to toluene and hexane vapour of various concentrations. The mass gain was found to increase monotonically with increasing vapour concentration up to a saturation level, indicating a bulk adsorption mechanism. It can be also seen from Fig. 2 that the adsorption of toluene vapour is more efficient than hexane. The number of adsorbed molecules per calixarene unit was found to be about 10 for hexane and 20 for toluene at the pressure of 0.9 PS. This corresponds well to the results obtained previously on the adsorption of benzene in C[4]RA LB films. 21 The numbers of adsorbed molecules found are much greater than those expected from the geometrical dimensions of the intrinsic calixarene cavity and the empty space between molecules. In order to explain this discrepancy, we have to assume either film swelling or condensation of vapour inside the film or both.
![The results of QCM measurements of P-C[4]RA LB films (20 layers) on exposure to toluene and hexane vapour of various concentrations.](/image/article/2000/JM/a903502h/a903502h-f2.gif) | ||
| Fig. 2 The results of QCM measurements of P-C[4]RA LB films (20 layers) on exposure to toluene and hexane vapour of various concentrations. | ||
Ellipsometric study
Further experimental evidence of vapour condensation, as well as film swelling, can be obtained using ellipsometry. These measurements were done using the vapour injection technique. Fig. 3 shows typical results of ellipsometric kinetic measurements for P-C[4]RA LB film (20 layers) during exposure to hexane vapour at different concentrations. The results consist of a very fast response followed by a similarly fast complete recovery on flushing with air. The origin of the initial sharp response observed will be discussed later. The value corresponding to the steady state of adsorption, achieved in 20–30 s, was found to depend on the concentration of hexane vapour. Very similar behaviour was observed in the case of toluene vapour. The readings of both the polariser and analyser angles were taken at the adsorption steady state condition, and the ellipsometric parameters Ψ and Δ were then calculated. Calculations of film thickness ( d) and refractive index ( n) were performed by numerically solving the reverse ellipsometric problem [ eqn. (3)] 30![The kinetics of the photodetector signal in ellipsometric measurements on P-C[4]RA LB films (20 layers) in response to injection of hexane vapour.](/image/article/2000/JM/a903502h/a903502h-f3.gif) | ||
| Fig. 3 The kinetics of the photodetector signal in ellipsometric measurements on P-C[4]RA LB films (20 layers) in response to injection of hexane vapour. | ||
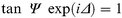 | (3) |
by using a least-squares fitting technique. In the above equation, Ψ and Δ are, respectively, the amplitude ratio and the phase shift of p- and s- components of polarised light reflected from the film surface. Reasonably thick LB films (20 layers) were used in these experiments in order to avoid thicknesses less than 10 nm, which leads to very poor resolution for the simultaneous determination of both n and d. A value of k = 0 for the extinction coefficient was used for these calculations since calixarene films are transparent at 632.8 nm.
The initial n and d values for P-C[4]RA LB films were found to be of 1.46 and 13.8 nm, respectively, which corresponds well to the results obtained previously for the LB films of unmodified C[4]RA. 21
Fig. 4 shows the changes of both the thickness and refractive index of LB films of P-C[4]RA in response to toluene and hexane vapours of various concentrations. The film thickness was found to increase monotonically with the vapour concentration confirming the assumption of film swelling. However, relative changes of the thickness, falling in the range of 10%, are still not enough to provide the room required for a few tens of guest molecules per one calixarene unit. Condensation of vapour inside the film should, therefore, be taken into consideration.
![The dependences of the optical parameters of P-C[4]RA LB films (20 layers) on the concentration of toluene and hexane vapours: (a) refractive index, (b) film thickness.](/image/article/2000/JM/a903502h/a903502h-f4.gif) | ||
| Fig. 4 The dependences of the optical parameters of P-C[4]RA LB films (20 layers) on the concentration of toluene and hexane vapours: (a) refractive index, (b) film thickness. | ||
As seen from Fig. 4, adsorption of toluene results in an increase of n from 1.46 (for unexposed LB film) to 1.49 on exposure to a nearly saturated vapour pressure. On the contrary, exposure to hexane vapour leads to a decrease in n value down to 1.43 at saturated pressure level. These results can be explained in terms of accumulation of liquid solvents within the film bulk. In the case of liquid toluene having n = 1.49, the effective refractive index of the film increases from its initial value 1.46 with an increase of toluene content. In contrast, accumulation of liquid hexane ( n = 1.37) leads to a decrease in the effective n value for calixarene LB films.
Similar results have been obtained recently on thin films of poly(dimethylsiloxane) using spectroscopic ellipsometry. 31 It has been found that exposure of the films to tetrachloroethane and toluene vapours causes an increase in refractive index, while cyclohexane, having n similar to that of the polymer, did not produce any significant changes.
SPR measurements
The adsorption of different organic vapours was studied using SPR measurements on gold-coated glass slides with LB films of Azo-C[4]RA deposited on them. Typical responses on exposure to benzene vapour at different concentrations are shown in Fig. 5. The kinetics of the response are similar to those observed here using ellipsometry (see Fig. 3) and also similar to results reported previously. 21,22 The features of these adsorption kinetics will be discussed later. Other vapours, such as toluene, p-xylene, aniline, hexane and chloroform, have also been studied and show similar behaviour.![The kinetics of SPR response of Azo-C[4]RA LB films (4 layers) to benzene vapour of various concentrations: 1)
pS, 2)
pS/10, 3)
pS/100, 4)
pS/1000, 5)
pS/10000.](/image/article/2000/JM/a903502h/a903502h-f5.gif) | ||
| Fig. 5 The kinetics of SPR response of Azo-C[4]RA LB films (4 layers) to benzene vapour of various concentrations: 1) pS, 2) pS/10, 3) pS/100, 4) pS/1000, 5) pS/10000. | ||
The values of the response for different vapours (in the form of relative changes of the reflected light intensity), corresponding to steady state conditions, are summarised in Fig. 6. Two important features of the adsorption isotherms presented in Fig. 6 should be identified. Firstly, all isotherms show Langmuir adsorption (typical of thin films of absorbent 32) over a wide range of concentrations up to 0.1 pS (with pS the saturated vapour pressure) with a sharp increase of the response at pressures of about 0.2 pS. The latter can be attributed to an effect similar to capillary condensation in porous absorbents. 32 Secondly, the relative response correlates well with the saturated vapour pressure ( pS) of the vapours studied in the following sequence: chloroform ( pS = 190 mmHg), hexane (160 mmHg), benzene (75 mmHg), toluene (20 mmHg), p-xylene (3 mmHg), aniline (< 0.5 mmHg).
![Isotherms of adsorption of different organic vapours in Azo-C[4]RA LB films obtained with SPR.](/image/article/2000/JM/a903502h/a903502h-f6.gif) | ||
| Fig. 6 Isotherms of adsorption of different organic vapours in Azo-C[4]RA LB films obtained with SPR. | ||
Capillary condensation phenomenon
All the results presented above, as well as those published earlier, 21,22 can be explained by the condensation of organic vapours in the nanoporous matrix of calixarene LB films. This phenomenon, usually observed in porous media, rests in the condensation of vapour inside the capillaries at pressures lower than their saturated pressure ( pS) at a certain temperature. 32 The change of pressure required for vapour condensation can be described by Kelvin's equation for cylindrical pores 32 [ eqn. (4)]: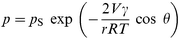 | (4) |
where p and pS are the saturated vapour pressure inside the capillaries with a radius r and in normal conditions, respectively. V is the molar volume and γ is the surface tension of the adsorbate and θ is the wetting angle in the capillary. This model can be extended to the case of nanoporous systems like the calixarene LB films with a characteristic pore size of about 1 nm. Knowing the parameters V and γ for particular adsorbates at room temperature and assuming full wetting of the pores by the solvents ( θ = 0), one can estimate p as 0.2–0.3 pS. This means that condensation of solvent vapours in calixarene LB films may start at pressures of about 0.2 pS, what is exactly observed in the current experiment (see Fig. 6).
The observed correlation between the values of the relative response and saturated vapour pressure becomes more understandable now, since more volatile solvents, having higher pS, will be condensed more easily. From this point of view, measuring vapour concentration in pS units seems to be more appropriate than in absolute values such as ppm. Simple calculations, based on an ideal gas approach, show for instance, that the saturation vapour of benzene is equivalent to 98000 ppm, compared to 4000 ppm for p-xylene and 800 ppm of aniline.
Adsorption kinetics
The results of the ellipsometric and the SPR kinetic measurements of vapour adsorption, shown, respectively, in Fig. 3 and 5, as well as earlier published data, have a characteristic initial sharp increase of the response followed by a decay and stabilisation after 20–30 s. The height of the initial peak depends on the concentration of the injected vapour, which indicates the link between this effect and vapour condensation phenomenon. However, there is a number of possible artefacts, which can influence the kinetics of the response. It has been shown earlier 21,22 by experiments on bare gold surfaces that light scattering on the turbulent vapour flow within the gas cell is negligible, as is condensation of vapour on gold surface. The leakage of vapour from the gas cell, as another possible artefact, has been ruled out due to extra care taken in sealing the gas cell. Condensation of vapour on the gas cell walls is also possible, and this could cause an undesirable decrease of vapour pressure inside the cell.Since the unusual kinetics were observed only in the case of using the vapour injection technique, two other methods of vapour preparation have been elaborated. Fig. 7 shows the SPR response of an Azo-C[4]RA LB film on exposure to saturated vapours of hexane, chloroform and toluene, formed within a gas cell by evaporation of a small droplet of the corresponding liquid solvent. The opposite type of kinetics observed here can easily be explained by the increasing vapour pressure (up to the saturation level) during the evaporation of liquid solvent. With this method it is difficult to control precisely the amount of injected liquid, which is required to form particular vapour concentrations lower than the saturated value.
![SPR response on exposure of Azo-C[4]RA LB films (4 layers) to saturated organic vapours formed by evaporation inside the gas cell.](/image/article/2000/JM/a903502h/a903502h-f7.gif) | ||
| Fig. 7 SPR response on exposure of Azo-C[4]RA LB films (4 layers) to saturated organic vapours formed by evaporation inside the gas cell. | ||
On the contrary, the method of running a constant vapour flow into the gas cell using a Standard Vapour Generator seems to provide the best option. The results obtained using this technique and presented in Fig. 8 show the absence of kinetics of both types, i.e. the sharp initial peak and the slow and gradual increase of the response. The response to a constant vapour flow is very fast as is the decay of the signal on flushing with a constant flow of nitrogen. A response time of about 10–12 s was found, much higher than a time of about 1 s required to fill the 2.5 cm 3 gas cell under a flow rate of 150 cm 3 min −1. Thus, the characteristic adsorption time constant is of the order of 10 s. It can be concluded therefore that the observed unusual kinetics of the response are due to the method of injection of a highly concentrated vapour. The mechanism of this kind of kinetics is caused, possibly, by the initial vapour condensation on the surface of the calixarene LB film with subsequent diffusion of the adsorbents inside the film matrix.
![SPR response of Azo-C[4]RA LB films (4 layers) to a constant flow of toluene vapour of concentration of 180 ppm.](/image/article/2000/JM/a903502h/a903502h-f8.gif) | ||
| Fig. 8 SPR response of Azo-C[4]RA LB films (4 layers) to a constant flow of toluene vapour of concentration of 180 ppm. | ||
Concluding remarks
Adsorption of several organic vapours, such as benzene, toluene, p-xylene, aniline, hexane and chloroform, in Langmuir–Blodgett films of two novel calix[4]resorcinarene derivatives were studied using QCM, ellipsometry and SPR experimental techniques. In line with the results obtained earlier for LB films of unmodified C[4]RA, fast and fully reversible response on exposure to high concentrations of organic solvent vapours has been established. The large number of adsorbed molecules per calixarene unit found with QCM measurements, along with the ellipsometry results, allow us to assume film swelling and accumulation of liquid solvent in the film bulk. Sublinearity of the adsorption isotherm, obtained with SPR, also indicates condensation of vapours within the film. It should be noted that the accumulation of liquid solvent within the calixarene film may make it less rigid and introduce non-linearity in the Δ m (Δ f) dependence for the QCM method. 29 However, the observed mass changes (see Fig. 3) of about 1 μg are well below the limit of linearity of QCM, which is about 100 μg for the quartz crystals used. The validity of using eqn. (1) is further supported by our earlier observation of the linear dependence of Δ m on the number of LB layers for C[4]RA films after exposure to saturated benzene vapour. 21 Analysis of the results obtained allows us to propose the following mechanism of adsorption of organic vapours in LB films of calix[4]resorcinarene derivatives. Calixarene LB films consist of a nanoporous matrix formed by the intrinsic calixarene cavities, and the empty spaces between the molecules and between the substituent alkyl chains. Organic vapours can penetrate through these pores inside the film matrix and condense there. This is accompanied by film swelling and changes in the film refractive index with respect to the refractive index of adsorbed liquid. Because the size of the pores is in the nanometre range, condensation of vapour may take place at pressures much lower than the corresponding saturated limit, particularly at 0.2 pS, according to the Kelvin equation [ eqn. (3)]. The proposed adsorption model does not contradict the conventional host–guest mechanism of molecular recognition, but complements it in the high vapour concentration range. Recent results of an SPR study of the exposure of thin spin coated films of seven different calixarene derivatives to a constant flow of four different vapours (benzene, toluene, ethylbenzene and p-xylene), produced by a standard vapour generator, show very good agreement with the proposed model. 33 In particular, a correlation between the SPR response and pS values of the vapour used was found there.The technique of vapour injection, used in the present study, as well as in our earlier publications, yields an additional sharp initial increase of the response, possibly due to initial vapour condensation on the film surface. The constant vapour flow technique eliminates this unusual kinetic behaviour and consequently would be preferred for further experiments.
Acknowledgements
The authors would like to acknowledge with gratitude the financial support received from EPSRC, UK and INCO-Copernicus, EU. The authors also wish to thank Professor V. I. Kalchenko, Dr A. Shivaniuk and Dr F. Davis for synthesis of amphiphilic calix[4]resorcinarene derivatives used in the present work.References
- Y. Kunugi, K. Nigorikawa, Y. Harima and K. Yamashita, J. Chem. Soc., Chem. Commun., 1994, 873 RSC.
- C. J. Lu and J. S. Shin, Anal. Chim. Acta, 1995, 306, 129 CrossRef CAS.
- Z. P. Deng, D. C. Stone and M. Thompson, Analyst, 1996, 121, 1341 RSC.
- B. P. J. D. Costello, P. Evans, R. J. Ewen, C. L. Honeybourne and N. M. Ratcliffe, J. Mater. Chem., 1996, 6, 289 RSC.
- E. Milella, F. Musio and M. B. Alba, Thin Solid Films, 1996, 285, 908 CrossRef.
- Z. K. Chen, S. C. Ng, S. F. Y. Li, L. Zhong, L. G. Xu and H. S. O. Chan, Synth. Met., 1997, 87, 201 CrossRef CAS.
- X. C. Zhou, L. Zhong, S. F. Y. Li, S. C. Ng and H. S. O. Chan, Sens. Actuators, B, 1997, 42, 59 CrossRef.
- Z. Cao, D. Cao, Z. G. Lei, H. G. Lin and R. Q. Yu, Talanta, 1997, 44, 1413 CrossRef.
- V. Papes and S. Brodska, Sens. Actuators, B, 1997, 40, 143 CrossRef.
- K. Buhlmann, B. Schladtt, K. Cammann and A. Shulga, Sens. Actuators, B, 1998, 49, 156 CrossRef.
- T. A. Dickinson, J. White, J. S. Kauer and D. R. Walt, Nature, 1996, 382, 697 CrossRef CAS.
- M. De Wit, E. Vanneste, H. J. Geise and L. J. Nagels, Sens. Actuators, B, 1998, 50, 164 CrossRef.
- G. Barko and J. Hlavay, Anal. Chim. Acta, 1998, 367, 135 CrossRef CAS.
- C. D. Gutsche, Calixarenes, Royal Society of Chemistry, Cambridge, UK, 1989. Search PubMed.
- D. J. Cram, S. Karbach, H-E. Kim, C. B. Knober, E. F. Maverick, J. L. Ericson and R. C. Hegelson, J. Am. Chem. Soc., 1988, 110, 2229 CrossRef CAS.
- F. L. Dickert, U. P. A. Baumler and G. K. Zwissler, Synth. Met., 1993, 61, 47 CrossRef CAS.
- P. Nelli, E. Delcanale, G. Faglia, G. Sberveglieri and P. Soncini, Sens. Actuators, B, 1993, 13-14, 302 CrossRef.
- K. D. Schierbaum, T. Weiss, E. U. Thoden van Velzen, J. F. J. Engbersen, D. N. Reinhoudt and W. Gopel, Science, 1994, 265, 1413 CrossRef.
- E. Dalcanale and J. Hartman, Sens. Actuators, B, 1995, 24, 39 CrossRef.
- J. Rickert, T. Weiss and W. Gopel, Sens. Actuators, B, 1996, 31, 45 CrossRef.
- A. V. Nabok, N. V. Lavrik, Z. I. Kazantseva, B. A. Nesterenko, L. N. Markovskiy, V. I. Kalchenko and A. N. Shivaniuk, Thin Solid Films, 1995, 259, 244 CrossRef CAS.
- A. V. Nabok, A. K. Hassan, A. K. Ray, O. Omar and V. I. Kalchenko, Sens. Actuators, B, 1997, 45, 115 CrossRef.
- F. L. Dickert, U. P. A. Baumler and H. Stathopulos, Anal. Chem., 1997, 69, 1000 CrossRef CAS.
- C. Granito, J. N. Wilde, M. C. Petty, S. Hoghton and P. J. Iredale, Thin Solid Films, 1996, 284-285, 98 CrossRef CAS.
- V. I. Kalchenko, D. A. Rudkevich, D. N. Shivaniuk, I. F. Tsymbal and L. N. Markovskiy, Zh. Obsch. Khim., 1994, 64, 731 (in Russian). Search PubMed.
- Yu. M. Shirshov, S. A. Zynio, E. P. Matsas, G. V. Beketov, A. V. Prokhorovich, E. F. Venger, L. N. Markovskiy, V. I. Kalchenko, A. V. Soloviov and R. Merker, Supramol. Sci., 1997, 4, 491 CrossRef CAS.
- O. Omar, A. K. Ray, A. K. Hassan and F. Davis, Supramol. Sci., 1997, 4, 417 CrossRef CAS.
- A. K. Hassan, A. V. Nabok, A. K. Ray, A. Lucke, K. Smith, S. J. M. Stirling and F. Davis, Mater. Sci. Eng., 1999, in the press. Search PubMed.
- D. S. Ballantine, R. M. White, S. I. Martin, A. J. Ricco, E. T. Zellers, G. C. Fry and H. Wohltjen, Acoustic Wave Sensors. Theory, Design, and Physico-Chemical Applications, Academic Press, New York, 1997. Search PubMed.
- A. M. A. Azzam and N. M. Bashara, Ellipsometry and Polarised Light, North Holland Publishing Co, Amsterdam, 1977. Search PubMed.
- K. Spaeth, G. Kraus and G. Gauglitz, Fresenius, J. Anal. Chem., 1997, 357, 292 CrossRef CAS.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press, London and New York, 1967. Search PubMed.
- A. K. Hassan, M. V. Molina, A. K. Ray, A. V. Nabok, Z. Ghassemlooy, R. Yates and R. Saatchi, Proceedings of SPIE's 6th Annual International Symposium on Smart Structures and Materials, 1–5 March, 1999, 3673. Search PubMed.
Footnote |
| † Basis of a presentation given at Materials Chemistry Discussion No. 2, 13–15 September 1999, University of Nottingham, UK. |
| This journal is © The Royal Society of Chemistry 2000 |
