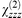Second-harmonic generation from alternate-layer and Z-type Langmuir–Blodgett films: optimisation of the transparency/efficiency trade-off†
Geoffrey J. Ashwell*, Rakesh Ranjan, Anne J. Whittam and Daniel S. Gandolfo
The Nanomaterials Group, Centre for Photonics and Optical Engineering, Cranfield University, Cranfield, UK MK43 0AL, g.j.ashwell@cranfield.ac.uk
First published on UnassignedUnassigned22nd December 1999
Abstract
Langmuir–Blodgett (LB) films of (E)-4-[(N-octadecyl-5,6,7,8-tetrahydro-5-isoquinolylidene)methyl]-N,N-dialkylaniline octadecyl sulfate, where the donor group is dimethylamino (1a), diethylamino (1b), dibutylamino (1c) and dihexylamino (1d), have optimum susceptibilities when the dyes are co-deposited in a 1∶1 mole ratio with octadecanoic acid. Alternate-layer structures of the mixed films, interleaved with poly(tert-butyl methacrylate), are non-centrosymmetric and the second-harmonic intensity increases with thickness as  to N>100 bilayers. Furthermore, when the dialkylamino group is sufficiently hydrophobic, i.e. for 1c and 1d, the dyes form non-centrosymmetric Z-type structures without interleaving spacer layers. The optimum susceptibilities, chromophore tilt angles, thicknesses and refractive indices of the interleaved and non-interleaved mixed films are as follows: alternate-layer (1b),
to N>100 bilayers. Furthermore, when the dialkylamino group is sufficiently hydrophobic, i.e. for 1c and 1d, the dyes form non-centrosymmetric Z-type structures without interleaving spacer layers. The optimum susceptibilities, chromophore tilt angles, thicknesses and refractive indices of the interleaved and non-interleaved mixed films are as follows: alternate-layer (1b),  = 67 pm V−1 at 1.064 µm for φ = 37°, l = 4.08 nm bilayer−1, nω = 1.50 and n2ω = 1.58; Z-type (1c),
= 67 pm V−1 at 1.064 µm for φ = 37°, l = 4.08 nm bilayer−1, nω = 1.50 and n2ω = 1.58; Z-type (1c),  = 76 pm V−1 for φ = 33°, l = 3.15 nm layer−1, nω = 1.52 and n2ω = 1.58. The moderately high susceptibilities arise from an optimised transparency/efficiency trade-off, the films in this series being transparent at the fundamental wavelength (1.064 µm) and having a very slight absorbance of 5 × 10−4 per active layer at 532 nm.
= 76 pm V−1 for φ = 33°, l = 3.15 nm layer−1, nω = 1.52 and n2ω = 1.58. The moderately high susceptibilities arise from an optimised transparency/efficiency trade-off, the films in this series being transparent at the fundamental wavelength (1.064 µm) and having a very slight absorbance of 5 × 10−4 per active layer at 532 nm.
Donor–(π-bridge)–acceptor materials have larger second-order nonlinear optical coefficients and provide an attractive alternative to their inorganic counterparts.1 Also, when substituted with a hydrophobic alkyl tail, the Langmuir–Blodgett (LB) technique may be used to fabricate non-centrosymmetric structures for second-harmonic generation (SHG). The molecules align at the air/water interface but, in the deposited film, tend to adopt a centrosymmetric arrangement with successive interfaces being hydrophilic (head-to-head) and hydrophobic (tail-to-tail).2 This may be overcome by alkylating both ends of the chromophore (CnH2n + 1-D–π–A-CmH2m + 1), thereby rendering the upper and lower surfaces hydrophobic,3–5 or by alternating the LB layers with complementary spacer materials, the interleaving molecules being hydrophobically substituted in the opposite sense:6–9
When both component layers are SHG-active and the interfacial interactions are sterically unhindered,2 the second-order properties are disadvantaged by the fact that the intralayer and interlayer dipoles are opposed. Passive spacers may be preferable10–14 but they have the drawback of diluting the optically nonlinear component in alternate-layer films. Thus, the interleaving layer should be carefully chosen and, for this purpose, poly(tert-butyl methacrylate) is an ideal candidate.15 It readily deposits on the downstroke and is, therefore, compatible with the preferred transfer of the active dye layer on the upstroke. Furthermore, it is transparent throughout the visible and near infrared regions of the spectrum and has a thickness of only 1.03 nm per layer. Penner et al.16,17 have reported low-loss waveguide structures, with an optical attenuation of 1 to 2 dB cm−1, for alternate-layer structures of polymeric dyes and poly(tert-butyl methacrylate), and have demonstrated efficient phase-matched blue light generation with a normalised conversion efficiency of 150% W−1 cm−2 for 819 nm radiation.
In this work, we report alternate-layer structures of a cationic hemicyanine dye interleaved with poly(tert-butyl methacrylate) and demonstrate a quadratic SHG dependence,  , to more than 100 bilayers. There have been several previous studies on interleaved films of hemicyanine derivatives, both monomeric11,12 and polymeric,13 but most have failed to show the theoretically expected SHG enhancement to thicknesses of more than a few bilayers.18,19 This suggests a structurally disordered arrangement and recently, Han et al.20 have reported a susceptibility enhancement from 16 pm V−1, when freshly deposited, to 50 pm V−1 when poled by a field of 1.3 MV m−1. The hemicyanine derivatives are also highly coloured and thus, combined with the difficulty of controlling the long-range structural order, they are not suitable for nonlinear optical applications.
, to more than 100 bilayers. There have been several previous studies on interleaved films of hemicyanine derivatives, both monomeric11,12 and polymeric,13 but most have failed to show the theoretically expected SHG enhancement to thicknesses of more than a few bilayers.18,19 This suggests a structurally disordered arrangement and recently, Han et al.20 have reported a susceptibility enhancement from 16 pm V−1, when freshly deposited, to 50 pm V−1 when poled by a field of 1.3 MV m−1. The hemicyanine derivatives are also highly coloured and thus, combined with the difficulty of controlling the long-range structural order, they are not suitable for nonlinear optical applications.
This paper concerns a subtle modification to the molecular structure of the cationic dye compared with the previously reported hemicyanine,11–13,18–20 the suitability of its counterion, and co-deposition with octadecanoic acid to optimise local field effects and absorption in the vicinity of the second-harmonic wavelength. LB films of the modified dyes (1a–d) show improved SHG when the anion is amphiphilic, i.e. octadecyl sulfate rather than iodide, and the intensity is enhanced when the dye is diluted in a 1∶1 ratio with octadecanoic acid. The second-harmonic intensity from alternate-layer films of the dye and poly(tert-butyl methacrylate) increases quadratically with the number of active layers and, furthermore, a similar dependence has been realised to in excess of 200 Z-type layers for analogues with hydrophobically substituted donor groups.
Experimental
Synthesis
To a solution of N-octadecyl-5,6,7,8-tetrahydroisoquinolinium iodide (0.51 g, 1 mmol) and 4-(N,N-dimethylamino)benzaldehyde (0.15 g, 1 mmol) in methanol (20 cm3) was added piperidine (2 drops) and the resultant mixture heated at reflux for 12 h. Upon cooling, a red microcrystalline product of the iodide salt of 1a was collected and recrystallised from methanol. Several batches were synthesised: typical yield ca. 70%. δH (200 MHz; CDCl3, J/Hz): 0.90 (t, J 6, 3H, CH3), 1.21 (br s, 32 H, CH2), 1.95–2.03 (m, 2H, CH2), 2.93–3.12 (m, 4H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-CH2), 3.13 (s, 6H, NCH3), 4.68 (t, J 6, 2H, CH2N+), 6.74 (d, J 4, 2H, Ar-H), 7.43 (s, 1H, C
C-CH2), 3.13 (s, 6H, NCH3), 4.68 (t, J 6, 2H, CH2N+), 6.74 (d, J 4, 2H, Ar-H), 7.43 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-H), 7.47 (d, J 4, 2H, Ar-H), 8.08 (d, J 4, 1H, Ar-H), 8.67 (d, J 4, 1H, Ar-H), 8.87 (s, 1H, Ar-H). UV–VIS (CHCl3): λmax, 487 nm. Found, C, 66.8; H, 8.8; N, 4.2%. C36H57N2I requires: C, 67.05; H, 8.91; N, 4.34%.
C-H), 7.47 (d, J 4, 2H, Ar-H), 8.08 (d, J 4, 1H, Ar-H), 8.67 (d, J 4, 1H, Ar-H), 8.87 (s, 1H, Ar-H). UV–VIS (CHCl3): λmax, 487 nm. Found, C, 66.8; H, 8.8; N, 4.2%. C36H57N2I requires: C, 67.05; H, 8.91; N, 4.34%.The diethylamino to dihexylamino analogues, 1b to 1d, were obtained in a similar manner by the reaction of N-octadecyl-5,6,7,8-tetrahydroisoquinolinium iodide and the appropriate 4-(N,N-dialkylamino)benzaldehyde, the NMR and elemental data being satisfactory in each case. Salts with amphiphilic anions were obtained by metathesis of the cationic dye and sodium octadecyl sulfate at the air/water interface of the LB trough (see below). The water soluble ions, Na+ and I−, dissolve into the aqueous subphase and are not incorporated into the film.
LB Deposition
Dilute solutions of the dye in chloroform and sodium octadecylsulfate in methanol were spread in a 1∶1 mole ratio onto the pure water subphase of one compartment of an alternate-layer LB trough (Nima Technology, model 622), left for 5 min and then compressed at 0.5 cm2 s−1 (ca. 0.1% s−1 of compartment area). The surface pressure versus area (π–A) isotherms of the four dyes show a limiting area of ca. 150 to 170 Å2 molecule−1 at π = 0, a broad plateau region and a steeply rising section with an area of ca. 40 Å2 molecule−1 just prior to collapse (e.g. see Fig. 1 for 1b). The data suggest that the chromophores initially reside face down on the aqueous subphase but are tilted upwards during compression, with the limiting area in the high-pressure regime corresponding to the sum of the van der Waals cross-sectional areas of the two octadecyl chains. | ||
| Fig. 1 Surface pressure versus area isotherm of 1b. | ||
Mixed films of the dye and octadecanoic acid were obtained in a similar manner, for example, by co-spreading the three components (dye∶Na+ODS−∶ODA) in a ratio of 1∶1∶n where 1 ≤ n ≤ 5. The general shape of the isotherm remains the same, when the dye is mixed with octadecanoic acid, but the combined area is progressively shifted by ca. 19 Å2 per ODA molecule for mole ratios of 1∶1 to 1∶5. Monolayer films were deposited onto hydrophilically treated glass substrates, on the upstroke, at a rate of 80 μm s−1 and at optimum surface pressures of 40 mN m−1 for 1a and 32 mN m−1 for 1b–d.
The inactive spacer, poly(tert-butyl methacrylate), used in the fabrication of the alternate-layer structures, was spread from dilute chloroform solution onto the water subphase of the second compartment of the trough. Interleaved films were then obtained by cycling a hydrophilically treated glass substrate (for SHG) or a silicon wafer (for reflectance studies), from below the surface, to deposit the mixed film (dye∶Na+ODS−∶ODA = 1∶1∶1) on the first upstroke, as above, and poly(tert-butyl methacrylate) on the subsequent downstroke at 10–12 mN m−1. Multilayer films were fabricated by repeating the process with a deposition rate of 80 μm s−1 in both directions. In contrast, multilayer Z-type structures of 1c and 1d were obtained on the upstroke by cycling the substrate via the air/water interface of the second compartment, in this case, with no floating layer.
SHG Measurements
The second-harmonic intensity was measured in transmission as the angle of incidence (Θ) of the laser beam (Nd∶YAG, λ = 1.064 μm, p-polarised), relative to the LB film, was altered from 0 to 75° and the polarisation of the fundamental beam was rotated using a half-wave plate. The SHG is negligible at normal incidence (e.g. see Fig. 2) and, therefore, its variation with the number of deposited layers was investigated at a fixed angle of 45°. The intensity was calibrated against the Maker fringe envelope of a Y-cut quartz reference (d11 = 0.5 pm V−1) and the data analysed as described previously.4b | ||
| Fig. 2 Typical dependence of the second-harmonic intensity on the angle of incidence of the laser beam, relative to the LB film, for the dyes in this series. The data correspond to a Z-type film of 1d. | ||
Results and discussion
Monolayer films
The linear and nonlinear optical properties of 1a to 1d are listed in Table 1. They exhibit significantly different susceptibilities but, from the SHG polarisation dependence, the chromophore tilt angles are quite similar. The second-harmonic wavelength barely overlaps the trailing edge of the absorption band and the different nonlinearities are attributed to slight variations in the resonant enhancement. The diethylamino and dibutylamino analogues exhibit the highest susceptibilities, = 100–120 pm V−1 at 1.064 μm, and these coincide with an absorption maximum at ca. 425 nm and a film absorbance, albeit very weak, of ca. 5 × 10−4 layer−1 at the harmonic wavelength. In contrast, lower susceptibilities of 50–70 pm V−1 for 1a and 1d relate to a slight blue shift of the absorption maximum to ca. 415 nm and a reduced absorbance of ca. 3 × 10−4 layer−1 at 532 nm.
= 100–120 pm V−1 at 1.064 μm, and these coincide with an absorption maximum at ca. 425 nm and a film absorbance, albeit very weak, of ca. 5 × 10−4 layer−1 at the harmonic wavelength. In contrast, lower susceptibilities of 50–70 pm V−1 for 1a and 1d relate to a slight blue shift of the absorption maximum to ca. 415 nm and a reduced absorbance of ca. 3 × 10−4 layer−1 at 532 nm.
| Dye | λmax/nm | Amax/layer−1 |
| φ/° |
|---|---|---|---|---|
| 1a | 417 | 3 × 10−3 | 70 | 32 |
| 1b | 425 | 5 × 10−3 | 120 | 30 |
| 1c | 425 | 5 × 10−3 | 100 | 31 |
| 1d | 415 | 4 × 10−3 | 50 | 30 |
The linear and nonlinear optical properties are also dependent upon the film composition and optimum SHG arises when the dyes are mixed in a 1∶1 ratio with octadecanoic acid. The behaviour is similar in each case and, therefore, we report the SHG dependence of the diethylamino analogue which is representative of the series. Films of 1b exhibit absorption maxima at 425 nm for the pure dye, 450 nm for the 1∶1 mixed film and 480 nm for dye∶ODA ratios of 1∶2 to 1∶5. The SHG polarisation dependence is independent of composition and, using the method of Kajikawa et al.,21 corresponds to a chromophore tilt angle of 30 ± 1° relative to the substrate normal. In contrast, the susceptibility is strongly dependent upon the ratio of dye to octadecanoic acid with an optimum value of 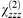 = 145 pm V−1 for the 1∶1 mixed film (cf. 120 pm V−1 for the pure dye). The enhancement is unlikely to be associated with resonance effects because the residual absorbance at the harmonic wavelength, albeit very small, is almost identical for each of the films of 1b. Therefore, it probably relates to improved non-centrosymmetric ordering and, as reported by Hayden22 and McGilp et al.,23 to changes in the local field effects upon dilution.
= 145 pm V−1 for the 1∶1 mixed film (cf. 120 pm V−1 for the pure dye). The enhancement is unlikely to be associated with resonance effects because the residual absorbance at the harmonic wavelength, albeit very small, is almost identical for each of the films of 1b. Therefore, it probably relates to improved non-centrosymmetric ordering and, as reported by Hayden22 and McGilp et al.,23 to changes in the local field effects upon dilution.
The films are almost transparent at 532 nm and thus, it may be assumed that Kleinman's symmetry is valid and that the susceptibility components are limited to  and
and  . Furthermore, as the hyperpolarisability is probably dominated by the component along the molecular charge transfer axis, the relation between χ(2) and β is as shown in eqn. (1) and (2).
. Furthermore, as the hyperpolarisability is probably dominated by the component along the molecular charge transfer axis, the relation between χ(2) and β is as shown in eqn. (1) and (2).
 | (1) |
 | (2) |
N is the number of molecules per unit volume, fω and f2ω are local field correction factors at ω and 2ω respectively, and f = (n2 + 2)/3 where n is the refractive index at the corresponding wavelength. The hyperpolarisability is suppressed in films with a high surface density of dye and, for 1b, saturates to a constant value of ca. 1.4 × 10−37 m4 V−1 (330 × 10−30 esu) when diluted with octadecanoic acid (Fig. 3). The apparent variation is attributed to changes in the local field correction factors and, interestingly, both β and λmax remain constant for dye∶ODA ratios of 1∶2 and above. However, as optimum SHG is realised for a dilution of 1∶1, the principal characterisation is limited to films of this composition and the following discussion only concerns this ratio.
 | ||
| Fig. 3 Variation of the second-order susceptibility (×) and molecular hyperpolarisability (filled circles) with the surface area occupied by 1b and associated ODA molecules in the mixed LB monolayers. The nonlinear optical coefficients were evaluated using a monolayer thickness of 3.1 nm and a weighted average of the refractive indices of the dye (nω = 1.51, n2ω = 1.60) and octadecanoic acid (nω = 1.53, n2ω = 1.54) as the ratio of dye∶ODA is altered from 1∶0 to 1∶5. | ||
Reflectance studies
The thickness and optical constants of films, deposited on silicon wafers, were determined using a four-layer model (air/LB/SiO2/Si) and a procedure similar to that described by Wang.24 Reflections from the multiple interfaces were analysed using a derivation based on the treatment of Heavens25 and applied to absorbing biaxial surfaces. The reflectance was determined as the angle of incidence of the laser beam, relative to the film, was rotated from 10° to 83° for both p and s polarised light. The films were investigated at the four wavelengths (green, 543.5 nm; yellow, 594.1 nm; orange, 611.9 nm; red, 632.8 nm) of a multiline He–Ne laser and the reflectance data fitted to theoretical equations using a Simplex curve fitting routine to minimise the sum of the least squares fit.The thickness and refractive indices of an alternate-layer mixed film of the diethylamino analogue (1b) were derived from an analysis of the wavelength dependent reflection data for a 32 bilayer film (Fig. 4; Table 2). A mean thickness of 130.8 nm corresponds to 4.08 nm bilayer−1 and, from studies on non-interleaved films of poly(tert-butyl methacrylate), conforms to ca. 3.05 nm layer−1 for the SHG-active dye and ca. 1.03 nm layer−1 for the spacer. The corresponding thickness from alternate-layer films of the dibutylamino analogue (1c) is 4.13 nm bilayer−1 and conforms to 3.10 nm layer−1 for the dye compared with 3.15 nm layer−1 obtained from the reflectance data of a non-interleaved Z-type film of 1c. Thus, for these analogues, a minimal difference in the layer thickness suggests that the dialkylamino groups are expanded in the horizontal rather than the vertical direction. Sufficient space is made available, at the other end, by a side-by-side packing arrangement of the three octadecyl tails of the cationic dye, the amphiphilic anion and the octadecanoic acid.
 | ||
| Fig. 4 Reflectance of polarised light from 32 bilayers of the 1∶1 mixed film of 1b and octadecanoic acid interleaved with poly(tert-butyl methacrylate) on a silicon wafer: (a) λ = 543.5 nm; (b) 594.1 nm; (c) 611.9 nm; (d) 632.8 nm. The curves represent the theoretical fits using the data in Table 2. | ||
| Coefficient | Wavelength/nm | |||
|---|---|---|---|---|
| 543.5 | 594.1 | 611.9 | 632.8 | |
| nx | 1.565 | 1.547 | 1.540 | 1.538 |
| ny | 1.557 | 1.526 | 1.520 | 1.515 |
| nz | 1.583 | 1.544 | 1.530 | 1.526 |
| niso | 1.568 | 1.539 | 1.530 | 1.526 |
| kx | 7.0 × 10−3 | 1.0 × 10−4 | 0 | 0 |
| ky | 0.3 × 10−3 | 1.7 × 10−4 | 0 | 0 |
| kz | 1.5 × 10−2 | 5.0 × 10−4 | 0 | 0 |
| l/nm | 130.5 | 130.4 | 130.8 | 130.6 |
| l/nm bilayer−1 | 4.078 | 4.075 | 4.088 | 4.081 |
The refractive indices, obtained from the analysis of the reflectance data of the alternate-layer film of 1b, increase with decreasing wavelength and their dispersion may be represented by eqn. (3), the Sellmeir equation (Fig. 5).
 | ||
| Fig. 5 Variation of the isotropic refractive index with wavelength of a 1∶1 mixed film of 1b and octadecanoic acid interleaved with poly(tert-butyl methacrylate). The points represent the experimental data from Table 2 and the solid line is the theoretical fit using the Sellmeir equation. | ||
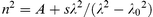 | (3) |
where A is a constant, s is the oscillator strength and λ0 is the resonance wavelength of the absorbance band. The refractive indices at the fundamental and harmonic frequencies, obtained by substitution of the experimental data into eqn. (3), are 1.495 and 1.581 respectively. The difference (n2ω − nω = 0.086) is smaller than reported26 for LiNbO3 and substitution into the following equation provides a coherence length of ca. 3 μm at normal incidence for the wavelength described in eqn. (4).
 | (4) |
The data obtained for the dibutylamino analogue (1c) are summarised in Table 3 for alternate-layer and Z-type films. The thickness and refractive indices of the alternate-layer structure are similar to those obtained for 1b.
| Dye | Dye∶ODA | Thickness/nm layer−1 | nω | n2ω | Film type |
|---|---|---|---|---|---|
| aCorresponds to the repeating bilayer structure of the alternate-layer film interleaved with poly(tert-butyl methacrylate). | |||||
| 1b | 1∶1 | 4.08a | 1.50 | 1.58 | interleaved |
| 1c | 1∶1 | 4.13a | 1.51 | 1.55 | interleaved |
| 1c | 1∶1 | 3.15 | 1.52 | 1.58 | Z-type |
| 1c | 1∶0 | 3.16 | 1.52 | 1.59 | Z-type |
SHG From alternate-layer films
Non-centrosymmetric multilayer structures are readily fabricated by interleaving the SHG-active layers with those of poly(tert-butyl methacrylate), the films showing a nearly quadratic increase in the second-harmonic intensity with the number of active layers. This was achieved to thicknesses in excess of 100 bilayers for the pure dyes, 1a to 1c, as well as for 1∶1 mixed films of the dyes and octadecanoic acid. Optimum intensities were obtained for the latter and, therefore, the following discussion is limited to the mixed LB films.The apparent quadratic SHG dependence of the 1∶1 mixed films of 1a and 1b is shown in Fig. 6 but, if viewed as  /N2vs.N, there are discrepancies in the normalised intensities. These probably result from variable deposition conditions. The multilayer structure was fabricated in several stages, throughout a period of two weeks, this tedious and unnecessary procedure resulting from our enforced requirement to monitor the linear and nonlinear optical behaviour throughout the entire deposition process. The diethylamino analogue (1b) exhibits the strongest SHG but the normalised intensity decreases by a factor of two throughout the early stages of deposition. However, it is constant for N > 55 bilayers and the deviation is also accompanied by a slight shift of the peak wavelength, from an initial value of 455 nm to 447 nm for thicknesses greater than 60 bilayers. Furthermore, the SHG polarisation dependence, I2ω(p → p)/I2ω(s → p), indicates that the chromophores modify their tilt from ca. 30° in the monolayer to 37° in the thick alternate-layer film, both values being relative to the normal to the substrate.
/N2vs.N, there are discrepancies in the normalised intensities. These probably result from variable deposition conditions. The multilayer structure was fabricated in several stages, throughout a period of two weeks, this tedious and unnecessary procedure resulting from our enforced requirement to monitor the linear and nonlinear optical behaviour throughout the entire deposition process. The diethylamino analogue (1b) exhibits the strongest SHG but the normalised intensity decreases by a factor of two throughout the early stages of deposition. However, it is constant for N > 55 bilayers and the deviation is also accompanied by a slight shift of the peak wavelength, from an initial value of 455 nm to 447 nm for thicknesses greater than 60 bilayers. Furthermore, the SHG polarisation dependence, I2ω(p → p)/I2ω(s → p), indicates that the chromophores modify their tilt from ca. 30° in the monolayer to 37° in the thick alternate-layer film, both values being relative to the normal to the substrate.
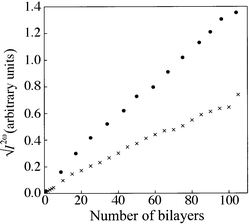 | ||
| Fig. 6 Variation of the square root of the second-harmonic intensity with the number of bilayers for 1∶1 mixed films interleaved with poly(tert-butyl methacrylate): 1a (×); 1b (solid circles). | ||
The second-order susceptibility decreases from 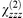 ≈ 110 pm V−1 for the first bilayer to a steady state of ca. 67 pm V−1 for structures in excess of 55 bilayers. Despite this, the properties are extremely encouraging and optimisation of the deposition conditions should result in improved second-order coefficients. The normalised SHG,
≈ 110 pm V−1 for the first bilayer to a steady state of ca. 67 pm V−1 for structures in excess of 55 bilayers. Despite this, the properties are extremely encouraging and optimisation of the deposition conditions should result in improved second-order coefficients. The normalised SHG,  , from the thick multilayer film is significantly greater than the signal obtained from LB films of the extensively studied hemicyanine, (E)-N-docosyl-4-[2-(4-dimethylaminophenyl)ethenyl]pyridinium bromide, first reported by Girling and co-workers.27 In addition, whereas the hemicyanine dye has an absorbance of ca. 0.002 layer−1 at 532 nm, the interleaved films of 1b, reported in this study, are almost transparent and have a corresponding absorbance of ca. 5 × 10−4 bilayer−1 (Fig. 7).
, from the thick multilayer film is significantly greater than the signal obtained from LB films of the extensively studied hemicyanine, (E)-N-docosyl-4-[2-(4-dimethylaminophenyl)ethenyl]pyridinium bromide, first reported by Girling and co-workers.27 In addition, whereas the hemicyanine dye has an absorbance of ca. 0.002 layer−1 at 532 nm, the interleaved films of 1b, reported in this study, are almost transparent and have a corresponding absorbance of ca. 5 × 10−4 bilayer−1 (Fig. 7).
 | ||
| Fig. 7 Visible spectrum of 104 bilayers of the 1∶1 mixed film of 1b interleaved with poly(tert-butyl methacrylate). The residual absorbance at the higher wavelength end of the spectrum is attributed to the glass substrate. | ||
Mixed films of the dimethylamino and dibutylamino analogues show a similar quadratic SHG dependence to more than 100 bilayers. Their linear and nonlinear optical properties are summarised in Table 4 . The former has a lower second-order susceptibility of 40 pm V−1 (cf. 67 pm V−1 for 1b). This coincides with a reduced absorbance of 3 × 10−4 bilayer−1 at the harmonic wavelength and the absorption maximum being shifted to 439 nm (Fig. 8). Thus, resonant effects can explain the altered nonlinear optical properties and, although the spectra probably reflect slightly different packing arrangements, it is unclear why they are dependent upon the dialkylamino groups. Nonetheless, when interleaved with poly(tert-butyl methacrylate), dyes in this series form non-centrosymmetric structures. They exhibit SHG comparable with that obtained from conventional hemicyanine derivatives18–20 but have a greatly improved transmittance at the harmonic wavelength.
 | ||
| Fig. 8 Visible spectrum of 105 bilayers of the 1∶1 mixed film of 1a interleaved with poly(tert-butyl methacrylate). | ||
| Dye | Dye∶ODA | λmax/nm |
| φ/° | Film type |
|---|---|---|---|---|---|
| 1a | 1∶0 | 410 | 26 | 39 | interleaved |
| 1b | 1∶0 | 420 | 50 | 33 | interleaved |
| 1c | 1∶0 | 425 | 31 | 32 | Z-type |
| 1d | 1∶0 | 410 | 21 | 30 | Z-type |
| 1a | 1∶1 | 439 | 40 | 30 | interleaved |
| 1b | 1∶1 | 447 | 67 | 37 | interleaved |
| 1c | 1∶1 | 450 | 52 | 33 | interleaved |
| 1c | 1∶1 | 450 | 76 | 33 | Z-type |
SHG From Z-type films
Unless interleaved, the dimethylamino and diethylamino analogues form centrosymmetric Y-type arrangements in which the molecules pack head-to-head and tail-to-tail. However, if the second end of the molecule is sufficiently hydrophobic, as in the case of the dibutylamino and dihexylamino analogues, a stable Z-type structure results. Quadratic enhancement of the second-harmonic intensity with the number of deposited layers has been observed for both 1c and 1d, the strongest SHG and most favourable packing being obtained for 1∶1 mixed films of the former (Fig. 9). In this case, quadratic SHG enhancement has been realised to in excess of 200 Z-type layers and, when carefully deposited, the normalised intensity,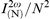 , matches the signal obtained from monolayer films. The Z-type films have a moderately high susceptibility of 76 pm V−1 for l = 3.15 nm, φ = 33°, nω = 1.52 and n2ω = 1.58 and, like other members of this series, are transparent at the fundamental wavelength and have a minimal absorbance of 5 × 10−4 layer−1 at the harmonic wavelength. The spectrum is almost identical with that of the 1∶1 mixed film of 1b (Fig. 7).
, matches the signal obtained from monolayer films. The Z-type films have a moderately high susceptibility of 76 pm V−1 for l = 3.15 nm, φ = 33°, nω = 1.52 and n2ω = 1.58 and, like other members of this series, are transparent at the fundamental wavelength and have a minimal absorbance of 5 × 10−4 layer−1 at the harmonic wavelength. The spectrum is almost identical with that of the 1∶1 mixed film of 1b (Fig. 7). | ||
| Fig. 9 Z-Type films of 1c: variation of the square root of the second-harmonic intensity with (a) the number of deposited layers and (b) the angle of incidence of the Nd∶YAG laser beam: p-polarised (solid circle) s-polarised (×) relative to the LB film. | ||
Another interesting feature of the 1∶1 mixed films of 1c and octadecanoic acid is that deposition on the downstroke (X-type) also results in non-centrosymmetric ordering but with the dipole orientation reversed. This has been demonstrated by X-type deposition onto a thick Z-type film, there being a suppression of the second-harmonic intensity when the number of layers of each type is equal. The intensity decreases quadratically with increasing film thickness and then, when the number of X-type layers is greater, it begins to increase quadratically. This is indicative of favourable LB deposition on the downstroke, as well as the upstroke, and may be used to fabricate waveguide structures with inversion symmetry in the thickness direction.16,28,29 The waveguiding properties of these films have not been studied but, in a parallel investigation on the 4-quinolinium analogue of 1c, we have previously reported Cerenkov-type phase matched SHG using fibre optic coupling.2,30 The materials reported here are more suitable candidates for investigation.
Conclusion
We conclude that the observed quadratic SHG dependence to more than 200 Z-type layers is a consequence of improved deposition and of our recent discovery that stable non-centrosymmetric structures result when the interface is invariably hydrophobic. The 200 layer film is the thickest Z-type structure to date and the normalised intensities, , obtained for interleaved films of the diethylamino analogue (1b) and Z-type films of the dibutylamino analogue (1c), both co-deposited with octadecanoic acid, are the strongest yet from semi-transparent LB films. The enhancement results, in part, from the proximity of the charge-transfer band which optimises the trade-off in efficiency and absorbance.
, obtained for interleaved films of the diethylamino analogue (1b) and Z-type films of the dibutylamino analogue (1c), both co-deposited with octadecanoic acid, are the strongest yet from semi-transparent LB films. The enhancement results, in part, from the proximity of the charge-transfer band which optimises the trade-off in efficiency and absorbance.Acknowledgements
The EPSRC (U.K.) is acknowledged for financial support of the nonlinear optics programme at Cranfield and for providing studentships to R.R., A.J.W. and D.G.References
- C. BosshardK. SutterP. PrêtreJ. HulligerM. FlörsheimerP. KaatzP. Günter, Organic Nonlinear Optical Materials, Advances in Optics, ed. A. F. Garito and F. Kajzar, Gordon and Breach, Basel, Switzerland, Vol. 1, 1995. Search PubMed.
- G. J. Ashwell, J. Mater. Chem., 1999, 9, 1991 RSC.
- G. J. Ashwell, P. D. Jackson and W. A. Crossland, Nature, 1994, 368, 438 CrossRef CAS.
- (a) G. J. Ashwell, G. Jefferies, C. D. George, R. Ranjan, R. B. Charters and R. P. Tatam, J. Mater. Chem., 1996, 6, 131 RSC; (b) G. J. Ashwell and R. Ranjan, Proc. SPIE Int. Soc. Opt. Eng., 1998, 3474, 76 Search PubMed.
- (a) G. J. Ashwell, P. D. Jackson, G. Jefferies, I. R. Gentle and C. H. L Kennard, J. Mater. Chem., 1996, 6, 137 RSC; (b) G. J. Ashwell, T. Handa and R. J. Ranjan, J. Opt. Soc. Am. B, 1998, 15, 466 Search PubMed.
- M. J. Roberts, G. A. Lindsay, K. J. Wynne, R. A. Hollins, P. Zarras, J. D. Stenger-Smith, M. Nadler and A. P. Chafin, Polym. Prepr., 1997, 38, 975 Search PubMed.
- H. R. Motschmann, T. L. Penner, N. J. Armstrong and M. C. Ezenyilimba, J. Phys. Chem., 1993, 97, 3933 CrossRef CAS.
- W. M. K. P. Wijekoon, S. K. Wijaya, J. D. Bhawalkar, P. N. Prasad, T. L. Penner, N. J. Armstrong, M. C. Ezenyilimba and D. J. Williams, J. Am. Chem. Soc., 1996, 118, 4480 CrossRef CAS.
- (a) G. A. Lindsay, K. J. Wynne, W. N. Herman, A. P. Chafin, R. A. Hollins, J. D. Stenger-Smith, J. Hoover, J. Cline and M. J. Roberts, Nonlinear Opt., 1996, 15, 139 Search PubMed; (b) G. A. Lindsay, M. J. Roberts, J. D. Stenger-Smith, W. N. Herman, P. R. Ashley and K. J. Wynne, Nav. Res. Rev., 1997, XLIX, 21 Search PubMed.
- (a) M. Era, K. Nakamura, T. Tsutsui, S. Saito, H. Niino, K. Takehara, K. Isomura and H. Tanaguchi, Jpn. J. Appl. Phys., 1990, 29, L2261 CAS; (b) M. Era, H. Kawafuji, T. Tsutsui, S. Saito, K. Takehara, K. Takehara, K. Isomura and H. Taniguchi, Thin Solid Films, 1992, 210–211, 163 CrossRef CAS.
- (a) G. J. Ashwell, E. J. C. Dawnay, A. P. Kuczynski and P. J. Martin, Proc. SPIE Int. Soc. Opt. Eng., 1991, 1361, 589 Search PubMed; (b) G. J. Ashwell, P. D. Jackson, D. Lochun, P. A. Thompson, W. A. Crossland, G. S. Bahra, C. R. Brown and C. Jasper, Proc. R. Soc. London A, 1994, 445, 385 Search PubMed.
- (a) S. H. Ma, K. Han, X. Z. Lu, W. C. Wang, Z. M. Zhang and Z. Q. Yao, Opt. Commun., 1994, 111, 513 CrossRef CAS; (b) S. Ma, X. Lu, J. Xu, W. Wang and Z. Zhang, J. Phys. D, 1997, 30, 2651 Search PubMed.
- (a) P. Hodge, Z. Ali-Adib, D. West and T. King, Macromolecules, 1993, 26, 1789 CrossRef CAS; (b) P. Hodge, Z. Ali-Adib, D. West and T. King, Thin Solid Films, 1994, 244, 1007 CrossRef CAS.
- (a) W. Hickel, B. Menges, O. Althoff, D. Lupo, U. Falk and U. Scheunemann, Thin Solid Films, 1994, 244, 966 CrossRef CAS; (b) I. Cabrera, A. Mayer, D. Lupo, U. Falk, U. Scheunemann and W. Hickel, Nonlinear Opt., 1995, 9, 161 Search PubMed.
- G. J. AshwellA. J. WhittamK. Skjonnemand, Langmuir, submitted for publication. Search PubMed.
- (a) T. L. Penner, H. R. Motschmann, N. J. Armstrong, M. C. Ezenyilimba and D. J. Williams, Nature, 1994, 367, 49 CrossRef CAS; (b) K. Clays, N. J. Armstrong, M. C. Ezenyilimba and T. L. Penner, Chem. Mater., 1993, 5, 1032 CrossRef CAS.
- (a) K. Clays, T. L. Penner, N. J. Armstrong and D. R. Robello, Proc. SPIE Int. Soc. Opt. Eng., 1992, 1775, 326 Search PubMed; (b) T. L. Penner, N. J. Armstrong, C. S. Willand, J. S. Schildkraut, D. R. Robello and A. Ulman, Nonlinear Opt., 1993, 4, 191 Search PubMed; (c) K. Clays, N. J. Armstrong and T. L. Penner, J. Opt. Soc. Am. B, 1993, 10, 886 Search PubMed.
- M. C. J. Young, W. X. Lu, R. H. Tredgold, P. Hodge and F. Abassi, Electron. Lett., 1990, 26, 993.
- L. Liu, J. Zheng, W. Wang, Z. Zhang, F. Tao, L. Xu and J. Hu, Opt. Commun., 1992, 93, 207 CrossRef CAS.
- K. Han, X. Lu, J. Xu, S. Ma and W. Wang, Opt. Commun., 1998, 152, 371 CrossRef CAS.
- K. Kajikawa, K. Kigata, H. Takezoe and A. Fukuda, Mol. Cryst. Liq. Cryst., 1990, 182, 91 Search PubMed.
- L. M. Hayden, Phys. Rev. B, 1988, 38, 3718 CrossRef CAS.
- J. F. McGilp, Z. R. Tang and M. Cavanagh, Synth. Met., 1993, 61, 181 CrossRef CAS.
- (a) H. Wang, Fiber Integr. Opt., 1994, 13, 293; (b) H. Wang, J. Modern Opt., 1995, 42, 497 Search PubMed; (c) H. Wang, J. Opt. Soc. Am. B, 1994, 11, 2331 Search PubMed.
- O. S. Heavens, Optical Properties of Thin Solid Films, 2nd rev. edn., Dover Publications, Mineola, 1991. Search PubMed.
- CRC Handbook of Laser Science and Technology, ed. M. J. Weber, CRC Press, Boca Raton, FL, 1982. Search PubMed.
- I. R. Girling, N. A. Cade, P. V. Kolinski, R. J. Jones, I. R. Peterson, M. M. Ahmad, D. B. Neal, M. C. Petty, G. G. Roberts and W. J. Feast, J. Opt. Soc. Am. B, 1987, 4, 950 Search PubMed.
- (a) N. Asai, H. Tamada, I. Fujiwara and J. Seto, J. Appl. Phys., 1992, 10, 4521 CrossRef; (b) I. Fujiwara, N. Asai and V. Howarth, Thin Solid Films, 1992, 221, 285 CrossRef CAS; (c) N. Asai, I. Fujiwara, H. Tamada and J. Seto, Mater. Res. Soc. Symp. Proc., 1994, 328, 91 Search PubMed.
- M. Küpfer, M. Flörsheimer, C. Bosshard and P. Günter, Nonlinear Opt., 1995, 10, 341 Search PubMed.
- S. Johal, S. W. James, R. P. Tatam and G. J. Ashwell, Opt. Lett., 1999, 24, 1194 Search PubMed.
Footnote |
| † Basis of a presentation given at Materials Chemistry Discussion No. 2, 13–15 September 1999, University of Nottingham, UK. |
| This journal is © The Royal Society of Chemistry 2000 |