Remediation of a nonachloro biphenyl congener with zero valent iron in subcritical water
Dirk C.
Hinz
ab,
Chien M.
Wai
b and
Bernd W.
Wenclawiak
*ab
aUniversität-GH Siegen, Analytische Chemie I, Adolf-Reichwein Str. 9, D-57068, Siegen
bUniversity of Idaho, Department of Chemistry, Moscow, ID 83844-2343, USA
First published on 1st February 2000
Abstract
Dechlorination of a nonachloro biphenyl congener with zero-valent iron in water under high temperature and pressure was investigated over time. Temperature has the main influence on the speed of dechlorination. Determination of polychlorinated biphenyls (PCBs) according to the grade of chlorination was performed by gas chromatography with mass selective detection in single ion monitoring mode. Dechlorination results in a variety of lower chlorinated biphenyls. The level of chlorination decreases with time. The amount of PCB molecules decreases to one-third within 90 min at 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 100 atm. However, no increase of biphenyl could be detected over time. A first-order kinetic model fitted the data obtained.
°C and 100 atm. However, no increase of biphenyl could be detected over time. A first-order kinetic model fitted the data obtained.
Introduction
Polychlorinated biphenyls (PCBs) are a group of compounds where one to ten hydrogen atoms of biphenyl are substituted by chlorine atoms. There are many applications of PCBs owing to their chemical and thermal stability and low or no inflammability. The production of PCBs started in 1929 in the USA. In 1976, the use of PCBs was restricted by the US Congress to only totally enclosed systems, such as electrical transformers and capacitors, due to their hazardous properties.1 Because of their stability, PCBs are still an environmental problem. PCBs in air, soil and water result from industrial discharge, emissions and natural transport mechanisms. For humans, as a final link in the food chain, the most important source of PCB intake is dietary fat originating from animals.2Incineration is one common way to destroy PCBs. However, at temperatures lower than 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C polychlorinated dibenzofurans (PCDFs) are formed.3 Other techniques can be used to dechlorinate PCBs. For example, Liu et al.4 described a titanium catalyst system based on sodium borohydride for the reduction of PCBs to biphenyl. The radiolytic degradation of nine PCB homologues in 2-propanol was reported by Arbon et al.,5 and the work of Zhang and Rusling6 demonstrated the electrochemical catalytic dechlorination of PCBs using bicontinuous microemulsions. Wang and Zhang7 described the remediation of PCBs with nanoscale iron particles in water at ambient temperatures as being only partial (<25% of the total mass) within 17 h. They obtained complete dechlorination within 17 h using palladized nanoscale iron. Chuang et al.8 described the dechlorination of most PCBs to biphenyl within 10 min at 400
°C polychlorinated dibenzofurans (PCDFs) are formed.3 Other techniques can be used to dechlorinate PCBs. For example, Liu et al.4 described a titanium catalyst system based on sodium borohydride for the reduction of PCBs to biphenyl. The radiolytic degradation of nine PCB homologues in 2-propanol was reported by Arbon et al.,5 and the work of Zhang and Rusling6 demonstrated the electrochemical catalytic dechlorination of PCBs using bicontinuous microemulsions. Wang and Zhang7 described the remediation of PCBs with nanoscale iron particles in water at ambient temperatures as being only partial (<25% of the total mass) within 17 h. They obtained complete dechlorination within 17 h using palladized nanoscale iron. Chuang et al.8 described the dechlorination of most PCBs to biphenyl within 10 min at 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C using Arochlor 1254. Arochlor 1254 contains mainly tetrachloro, pentachloro and hexachloro congeners. For the dehalogenation to biphenyl, water is the hydrogen donor.
°C using Arochlor 1254. Arochlor 1254 contains mainly tetrachloro, pentachloro and hexachloro congeners. For the dehalogenation to biphenyl, water is the hydrogen donor.
Matheson and Tratnyek9 studied the mechanism of the reductive dehalogenation of chlorinated methane by iron metal. They found that the reduction mechanism could be represented by eqn. (1). They found no evidence for a reductive dehalogenation by Fe2+, as a result of metal corrosion, or for a reduction by H2, formed by reduction of H2O during corrosion.
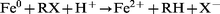 | (1) |
Cheng et al.10 proposed a mechanism of reductive dehalogenation by the catalysed hydrogenolysis by H2 as an important pathway for reductive dehalogenation. The mechanism can be described by eqns. (2) and (3).
 | (2) |
 | (3) |
Wai and coworkers11 reported the reduction of Arochlor 1260 with zero-valent iron in subcritical water as used in this paper. Reduction of PCBs to biphenyl in water is an ecologically beneficial method for the disposal of PCBs, since biphenyl is used as a preservative for citrus fruits. To study the dechlorination of PCBs in more detail, we decided to investigate the remediation of a nonachloro congener. We expected to observe each individual dechlorination step.
Experimental
The iron was cleaned by exposing 50 g of iron powder (Fisher Scientific, Fair Lawn, NJ, USA; <100 mesh) to 500 ml 1 M HCl for 1 h in a flask. Afterwards the iron was washed five times with deoxygenated (purged with nitrogen) and deionized water. The water was removed by washing three times with 50 mL of acetone. During each washing process the flask was purged with nitrogen. The clean and dry iron was washed with 50 mL of hexane twice to remove any organic contaminants. The remaining hexane was evaporated afterwards under a stream of nitrogen.A solution of 200 µL of 2,2′,3,3′,4,4′,5,6,6′-nonachloro biphenyl (ChemService, West Chester, PA, USA; BZ#20712) in n-hexane (Fisher Scientific) (500 ppm) was pipetted onto 2.500 g of previously cleaned iron. After hexane was evaporated, the spiked iron was mixed and placed in a 3 mL stainless-steel high pressure extraction cell (Keystone Scientific, Bellefonte, PA, USA). The cell was filled completely with deoxygenated water (HPLC grade, Fisher Scientific). The cell was connected to an apparatus shown in Fig. 1. Depending on which needle valve is closed, either hexane or water can flush the system. During heating and remediation time, the valve of the hexane pump Vp3 (see Fig. 1) was closed and the valve of the water pump Vp2 was open.
 | ||
| Fig. 1 Schematic diagram of the remediation apparatus. | ||
Before the system was heated, all tubing was filled with water to remove the air. The pressure was increased to 100 atm and the oven was heated to 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Pressure in the remediation cell was controlled and held constant by the water pump. After 15 min, the system reached its final temperature of 250
°C. Pressure in the remediation cell was controlled and held constant by the water pump. After 15 min, the system reached its final temperature of 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. This 15 min are defined as the heating time in the following. After this 15 min, the remediation time began. When a defined remediation time had passed, the needle valve Vo between the cooling loop and the outlet was opened. The solution flowed into a cooled vial, filled with 3 mL of hexane and 100 µL of a 540 ppm solution of decachloro biphenyl (ChemService; BZ#20912) in hexane. Decachloro biphenyl was used as internal standard. Water flushed the cell at about 3 mL min−1. About 7 mL of water phase was collected. Then, the valve Vp2 of the water pump was closed and the valve Vp3 for hexane was opened. About 7 mL of hexane solution was collected within 3–4 min. After this extraction of the cell with hexane, 3–4 mL of water was flushed once again through the cell. All solutions were collected in one vial. Hexane was separated and the water phase was extracted three times with 3 mL of hexane. All collected hexane phases were combined and concentrated to 10.0 mL by a stream of nitrogen.
°C. This 15 min are defined as the heating time in the following. After this 15 min, the remediation time began. When a defined remediation time had passed, the needle valve Vo between the cooling loop and the outlet was opened. The solution flowed into a cooled vial, filled with 3 mL of hexane and 100 µL of a 540 ppm solution of decachloro biphenyl (ChemService; BZ#20912) in hexane. Decachloro biphenyl was used as internal standard. Water flushed the cell at about 3 mL min−1. About 7 mL of water phase was collected. Then, the valve Vp2 of the water pump was closed and the valve Vp3 for hexane was opened. About 7 mL of hexane solution was collected within 3–4 min. After this extraction of the cell with hexane, 3–4 mL of water was flushed once again through the cell. All solutions were collected in one vial. Hexane was separated and the water phase was extracted three times with 3 mL of hexane. All collected hexane phases were combined and concentrated to 10.0 mL by a stream of nitrogen.
Quantification of PCBs is always a problem. Since it is a time-consuming and expensive method to calibrate with all 209 congeners, quantification has to be performed with selected congeners.13–16 For quantification, a PCB mixture in acetone containing eight congeners from mono- to octachloro biphenyl (100 µg mL−1; ChemService) and the nonachloro PCB BZ#207 (99%; ChemService) were used. n-Hexane (Fisher Scientific) was used as solvent. The compounds are listed in Table 1. Decachloro biphenyl (99.8%; ChemService) was added to each standard solution as internal standard. The concentration of the internal standard was 5.40 mg L−1 in each solution.
| Chlorine group | Quantification compounds | Detected ions | Retention time/min |
|---|---|---|---|
| Mono | 2-Chlorobiphenyl | 188, 152, 76 | 15.02 |
| Di | 2,3-Dichlorobiphenyl | 222, 152, 75 | 17.72 |
| Tri | 2,4,5-Trichlorobiphenyl | 256, 186, 150 | 19.84 |
| Tetra | 2,2′,4,4′-Tetrachlorobiphenyl | 292, 220, 110 | 21.64 |
| Penta | 2,2′,3′,4,6-Pentachlorobiphenyl | 326, 254, 184 | 23.45 |
| Hexa | 2,2′,4,4′,5,6′-Hexachlorobiphenyl | 360, 290, 145 | 25.78 |
| Hepta | 2,2′,3,3′,4,4′,6-Heptachlorobiphenyl | 394, 324, 162 | 31.07 |
| Octa | 2,2′,3,3′,4,5′,6,6′-Octachlorobiphenyl | 430, 358, 179 | 30.85 |
| Nona | 2,2′,3,3′,4,4′,5,6,6′-Nonachlorobiphenyl | 464, 394, 196 | 43.92 |
| Deca | 2,2′,3,3′,4,4′,5,5′,6,6′-Decachlorobiphenyl | 498, 428, 214 | 38.31 |
PCBs are sufficiently volatile and thus usually separated by gas chromatography. The most frequently used detection technique for PCBs is electron-capture detection (ECD). It is very sensitive and selective, but only one dimensional (Cl versus t) for halogenated compounds.17,18 For secure quantification and identification of individual PCB congeners, a two column set-up with known retention times of each congener on each column and careful calibration are required. A mass spectrometer is a multidimensional detection system (TIC versus t; mass versus t). Individual but characteristic masses of each analyte can be selected and identified.
Analysis of the extracts was performed by gas chromatography (HP 5890 Series II) with mass selective detection (HP 5971) in selected ion monitoring (SIM) mode. The sensitivity in the SIM mode is comparable to an ECD, but less information is obtained compared with the scan mode. The selected and recorded masses are shown in Table 1. Since the extracts contained only PCBs, identification was possible by comparison of the ratio of three ions each.
One microlitre of the hexane extracts was injected into a 30 m × 0.25mm × 0.25 µm SIL-8 CB-MS (Chrompack) column. The GC oven initial temperature was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for 1 min; this was increased at a rate of 10
°C for 1 min; this was increased at a rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 to 200
°C min−1 to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, then at a rate of 4
°C, then at a rate of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 to 300
°C min−1 to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, and maintained at this temperature for 5 min.
°C, and maintained at this temperature for 5 min.
Results and discussion
The chromatograms of reduced BZ#207 (with BZ#209 as internal standard) obtained at different temperatures and pressures are shown in Fig. 2. The remediation time of 40 min was kept constant in all three cases. The less chlorinated biphenyls have shorter retention times. The remediation of BZ#207 is further advanced if more peaks have shorter retention times. The term “up to 300 atm" in Fig. 2 means that the valve of the water pump was closed after the 15 min heating time. The pressure increased to 300 atm during the following 40 min of remediation time due to the formation of hydrogen gas. As Fig. 2 shows, the temperature influence (top and middle chromatogram) on the reduction of the nonachloro biphenyl to less chlorinated biphenyls is stronger than the pressure (middle and bottom chromatogram) influence. Therefore further remediation experiments were conducted at 100 atm and 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
°C.
 | ||
| Fig. 2 Gas chromatogram after remediation of BZ#207 at different temperatures and pressures. The remediation time in each case is 40 min. For definition of “up to 300 atm", see text. | ||
Fig. 3 shows the kinetics for the remediation of 2,2′,3,3′,4,4′,5,6,6′-nonachloro biphenyl. The heating period of 15 min is represented as negative values on the x-axis. It is notable that the nonachloro biphenyl congener is already reduced to less chlorinated biphenyls during the heating time. As Fig. 2 shows, BZ#207 is completely remediated only at 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. After 40 min of remediation time at 100
°C. After 40 min of remediation time at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, BZ#207 is still present. Obviously, as Fig. 3 shows, a temperature rise up to 250
°C, BZ#207 is still present. Obviously, as Fig. 3 shows, a temperature rise up to 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C within 15 min is sufficient to reduce almost all BZ#207.
°C within 15 min is sufficient to reduce almost all BZ#207.
 | ||
| Fig. 3 Kinetics of reduction for the nonachloro biphenyl. | ||
Adding the determined quantities of all PCBs at each investigated remediation time yields the plot of Fig. 4. The total amount of PCB molecules in the system decreases with time. The level of chlorination for the main isomer group decreases with time. At a remediation time of zero minutes (by definition when 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C was reached), the predominant PCB species is the heptachloro biphenyl. Ten minutes later it is the hexachloro biphenyl and, after a remediation time of 30 min, the biggest isomer group is the pentachloro biphenyl. After 40 min the main group contains the tetrachloro isomers.
°C was reached), the predominant PCB species is the heptachloro biphenyl. Ten minutes later it is the hexachloro biphenyl and, after a remediation time of 30 min, the biggest isomer group is the pentachloro biphenyl. After 40 min the main group contains the tetrachloro isomers.
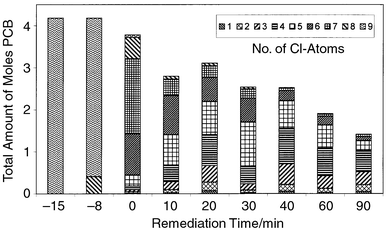 | ||
| Fig. 4 Total amount of PCBs at different remediation times. | ||
During 90 min at 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 100 atm, the molarity decreases to one third of its starting value. After 90 min remediation time of the nonachloro biphenyl congener, the solution contains several newly formed congeners from mono- to heptachloro biphenyls. The main isomer group contains tetrachloro biphenyls.
°C and 100 atm, the molarity decreases to one third of its starting value. After 90 min remediation time of the nonachloro biphenyl congener, the solution contains several newly formed congeners from mono- to heptachloro biphenyls. The main isomer group contains tetrachloro biphenyls.
The kinetic rate law for a reaction of first order is given in eqn. (4). Integration leads to eqn. (5).
 | (4) |
 | (5) |
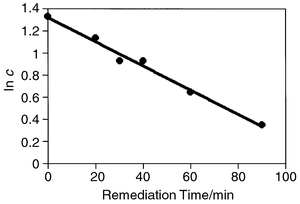 | ||
| Fig. 5 Plot of ln c versus remediation time. c = total moles PCB per litre of solution. | ||
Measurements of pH in different systems show that the pH increases slightly when iron and water react at 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 100 atm for 60 min. This confirms that eqn. (2) is correct. The surface colour of the iron changes to black due to the transformation of iron to magnetite in these conditions. Since the concentration of Fe2+ and Fe3+ in the reaction water was very low, we postulate the mechanism given in eqns. (6) to (8) to ; eqn. (7) is known as the Schikorr reaction and occurs at temperatures greater than 373 K.19
°C and 100 atm for 60 min. This confirms that eqn. (2) is correct. The surface colour of the iron changes to black due to the transformation of iron to magnetite in these conditions. Since the concentration of Fe2+ and Fe3+ in the reaction water was very low, we postulate the mechanism given in eqns. (6) to (8) to ; eqn. (7) is known as the Schikorr reaction and occurs at temperatures greater than 373 K.19
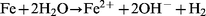 | (6) |
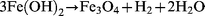 | (7) |
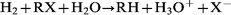 | (8) |
During the reaction of iron with BZ#207 in water at 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 100 atm for 60 min, the pH decreases. This observation corresponds well with eqn. (3).
°C and 100 atm for 60 min, the pH decreases. This observation corresponds well with eqn. (3).
To investigate the remediation products in more detail, solutions were analysed by mass selective detection in full scan mode. It was not possible to identify other remediation products. Biphenyl (m/z = 154) was also detected in SIM mode. During remediation over time, the concentration of biphenyl did not increase although the total molarity of PCBs decreased, as observed by Chuang et al.8 and confirmed by our data. An experiment with iron and biphenyl in water at 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and 100 atm for 60 min did not result in the decomposition of biphenyl. This could indicate that radicals are needed for the decomposition of biphenyl, e.g. chlorine radicals from the dechlorination process, and that the decomposition results in volatile compounds.
°C and 100 atm for 60 min did not result in the decomposition of biphenyl. This could indicate that radicals are needed for the decomposition of biphenyl, e.g. chlorine radicals from the dechlorination process, and that the decomposition results in volatile compounds.
Acknowledgements
DCH and BWW wish to thank the Department of Chemistry at the University of Idaho, for support during their stay there. DCH also wishes to thank the Fonds der Chemischen Industrie (Frankfurt, Germany) for financial support with a fellowship.References
- A. L. Alford-Stevens, Environ. Sci. Technol., 1986, 20, 1194 CAS.
- DFG, Deutsche Forschungsgemeinschaft, Polychlorierte Biphenyle: Bestandsaufnahme über Analytik, Vorkommen, Kinetik und Toxikologie, VCH, Weinheim, 1988. Search PubMed.
- M. D. Erickson, S. E. Swanson, J. D. Flora and G. D. Hinshaw, Environ. Sci. Technol., 1989, 23, 462 CAS.
- Y. Liu, J. Schwartz and C. L. Cavallaro, Environ. Sci. Technol., 1995, 29, 836 CAS.
- R. E. Arbon, B. J. Mincher and W. B. Knighton, Environ. Sci. Technol., 1994, 28, 2191 CAS.
- S. Zhang and J. F. Rusling, Environ. Sci. Technol., 1995, 29, 1195 CAS.
- C. B. Wang and W. X. Zhang, Environ. Sci. Technol., 1997, 31, 2154 CrossRef CAS.
- F. W. Chuang, R. A. Larson and M. S. Wessman, Environ. Sci. Technol., 1995, 29, 2460 CAS.
- L. J. Matheson and P. G. Tratnyek, Environ. Sci. Technol., 1994, 28, 2045 CAS.
- I. F. Cheng, Q. Fernando and N. Korte, Environ. Sci. Technol., 1997, 31, 1074 CrossRef CAS.
- H. K. Yak, B. W. Wenclawiak, I. F. Cheng, J. G. Doyle and C. M. Wai, Environ. Sci. Technol., 1999, 8, 1307 CrossRef CAS.
- K. Ballschmiter and M. Zell, Fresenius' J. Anal. Chem., 1980, 302, 20 CAS.
- G. P. Mertelli, M. G. Castelli and R. Fanelli, Biomed. Mass Spectrom., 1981, 8, 347 Search PubMed.
- A. L. Alford-Stevens, T. A. Bellar, J. W. Eichelberger and W. L. Budde, Anal. Chem., 1986, 58, 2014 CrossRef CAS.
- R. S. Collard and M. M. Irwin, Talanta, 1983, 30, 811 CrossRef CAS.
- G. W. Tindall and P. E. Wininger, J. Chromatogr., 1980, 196, 109 CrossRef CAS.
- S. D. Cooper, M. A. Moseley and E. D. Pellizzari, Anal. Chem., 1985, 57, 2469 CrossRef CAS.
- M. D. Mullin, C. M. Pochini, S. McCrindle, M. Romkes, S. H. Safe and L. M. Safe, Environ. Sci. Technol., 1984, 18, 468.
- G. Schikorr, Z. Elektrochim., 1929, 35, 62 Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
