Phase separation of polyelectrolytes and non-ionic polymers in frozen solutions
Ken-ichi Izutsu* and Kojima Shigeo
National Institute of Health Sciences, 1-18-1 Kamiyoga, Setagaya, Tokyo, 158-8501, Japan. E-mail: izutsu@nihs.go.jp
First published on UnassignedUnassigned19th January 2000
Abstract
The miscibility of polyelectrolytes and non-ionic polymers in frozen aqueous solutions was studied using diethylaminoethyl (DEAE)-dextran, dextran sulfate, poly(acrylic acid, sodium salt) (PAANa), Ficoll, polyvinylpyrrolidone (PVP) and dextran as model polymers. Thermal analysis of single-polymer frozen solutions showed a glass transition of maximally concentrated solution at particular temperatures [Tg′: equivalent to softening temperature of the amorphous phase (Ts)]. Combinations of polymers showed either single (e.g., PAANa and dextran, DEAE-dextran and dextran) or double (e.g., PAANa and Ficoll or PVP) Tg′s, representing concentrated polymer mixtures and separated phases. The addition of salts altered the miscibility of some polyelectrolyte and non-ionic polymer combinations in frozen solutions. Freeze-concentration separates DEAE-dextran and dextran (5% w/w each, into two phases in the presence of low-concentration (20–50 mM) NaCl, NaSCN, sodium phosphate buffer or TRIS–HCl buffer in initial solutions. A high concentration (300 mM) of salting-in salt (NaSCN) merged the two Tg′s, indicating the inhibition of phase separation. Salts in some frozen solutions are distributed differently to separated phases (dextran sulfate and Ficoll or PVP), which shifts the Tg′s independently. The miscibility of polyelectrolytes and non-ionic polymers in frozen aqueous solutions varies depending on the nature of the polymers and the existence of co-solutes. Salts should change some polymers' miscibility by concealing the electrostatic effect that favors molecular interactions.
Introduction
Freezing of an aqueous solution concentrates solutes among ice crystals. Solutes in multi-solute solutions are concentrated in an amorphous mixture phase or separated into different phases, depending on their miscibility.1–4 Some combinations of non-ionic polymers [e.g., polyvinylpyrrolidone (PVP) and dextran] or a polymer and a salt (e.g., PVP and sodium phosphate) separate into different amorphous phases in a frozen solution. Particular solutes [e.g., NaCl and poly(ethylene glycol) (PEG)] tend to crystallize in the separated phases.2,5 A better understanding of this phenomenon, from a practical point of view, is important in various areas, including the freeze-preservation of biological systems and the development of freeze-dried biopharmaceuticals.An unfavorable interaction between the hydrated solutes and the concentration during freezing can cause phase separation in frozen solutions.3 An aqueous solution containing various combinations of polymers or a polymer and a salt separates into two layers above critical concentrations at ambient temperature owing to the repulsive force between concentrated solutes and the excluded volume effect.6–8 This type of aqueous two-layer system has been used for partitioning biological macromolecules and particles. Crystallization of ice raises the polymer concentration in the non-ice amorphous phase as high as 75% w/w regardless of their initial concentrations.9 The freeze-concentration separates the combinations of unfavorably interacted solutes even from dilute single-phase solutions by raising the solute concentrations above the binodal curve of the systems. This makes the relative concentration of solutes in the initial solution an important factor in determining their miscibility in frozen solutions.4
Many solutes possess their intrinsic physical characteristics in frozen solutions. Thermal analysis of a single-solute frozen solution with a freeze-concentrated amorphous phase shows two thermal transitions that can be observed as changes in the heat capacity.10–12 The lower temperature transition is the ‘‘glass transition (Tg)’’ at which the amorphous phase changes from a glassy to a rubbery state. The higher temperature transition is usually more apparent in thermograms; however, there is some disagreement regarding the interpretation of the phenomenon.11,12 The transition is referred to as the ‘‘glass transition of the maximally freeze-concentrated solution (Tg′)’’ or the ‘‘softening temperature of the amorphous phase in frozen solution (Ts)’’. We employ the commonly used term ‘‘Tg′’’ in this paper. Crystallizing solutes often show a devitrification peak or a eutectic crystal melting peak in thermograms.
Thermal analysis of multi-solute solutions provides information on the solute miscibility in frozen solutions. While a miscible combination of polymers shows a single Tg′ of the mixed phase, a phase separation of polymers gives two Tg′s at temperatures close to their individual Tg′s. Some combinations of non-ionic polymers (e.g., PVP and dextran or Ficoll and dextran) separate in freezing single-phase initial solutions.4 The polymer miscibility depends largely on the co-solutes in the system. A high concentration of salting-in salt (e.g., NaSCN) prevents the phase separation between PVP and dextran in a frozen solution.4
Since little is known about the miscibility of polyelectrolytes and non-ionic polymers in frozen solutions, it is an interesting area of study. First, the interaction between polyelectrolytes and non-ionic polymers is very different from that in non-ionic polymer combinations. The contribution of an electrostatic effect favorable to polymer interaction makes many polyelectrolyte–non-ionic polymer combination solutions miscible at room temperature. The existence of salts has an important effect on the phase equilibrium because it masks the electrostatic effect.13 Some polymer combinations [e.g., dextran sulfate and PEG or PVP, diethylaminoethyl (DEAE)-dextran and poly(vinyl alcohol) (PVA)] form aqueous two-layer solutions in the presence of NaCl.7,8
Since many biological polymers, including proteins and polynucleotides, are polyelectrolytes, information about the polymer miscibility should help us to understand the physical behavior of biological polymers, supermolecular structures and organisms in frozen solutions. Recent advances in biotechnology require the rational design of freeze-dried biopolymer pharmaceuticals with appropriate stabilizing excipients. Some examples have suggested that the miscibility with a protein can determine the stabilizing effect of excipients. Phase separation and crystallization of excipients in a frozen solution not only deprives them of the biopolymer-protecting effect, but also the interface between the phases can damage the macromolecular structure.3,14–16
In this work, we studied the miscibility of polyelectrolytes and non-ionic polymers in frozen solutions and the effect of co-solutes on the systems through thermal analysis using DEAE-dextran, dextran sulfate, poly(acrylic acid, sodium salt) (PAANa), dextran, Ficoll and PVP as model polymers.
Materials and methods
DEAE-dextran [chloride form, average molecular weight (MW) 500000], dextran sulfate (sodium salt, average MW 10000), dextran (Leuconostoc mesenteroides, average MW 37500), PVP (average MW 40000) and Ficoll (type 70, average MW 70000) were purchased from Sigma Chemical. PAANa (average MW 30000, 40% solution) was obtained from Aldrich Chemical. Sodium thiocyanate, sodium chloride, sodium sulfate and sucrose were products of Wako Pure Chemical.Polymers, except dextran sulfate, were dialyzed against distilled water for 1 d and then freeze-dried. Polymer concentrations were determined from the weight of the freeze-dried solids. Thermal analysis of the frozen solutions was performed using a differential scanning calorimeter (TA Instrument, DSC 2920). Aliquots (10 μl) of solutions in aluminum cells were cooled to −100 °C and scanned at 5 °C min−1. The peak temperature of the derivative thermogram was determined as the Tg′ of the frozen solution. Sample solutions for the thermal analysis contained 5% w/w of each polymer unless stated otherwise. Solutions employed for the thermal analysis were single phase at room temperature.
Results and discussion
(1) Miscibility of polyelectrolytes and non-ionic polymers
The miscibility of polyelectrolytes and non-ionic polymers was studied by thermal analysis of frozen solutions containing single polymers or combinations of polymers. Single-polymer frozen solutions containing 5% w/w PAANa, DEAE-dextran, dextran sulfate, PVP, Ficoll or dextran showed Tg′ at −36.2, −27.1, −22.0, −22.0, −22.3 and −13.3 °C, respectively. The transition temperatures obtained were consistent with those found in the literature.11,17 As stated in the Introduction, the intrinsic nature of the transition is a subject of disagreement in recent literature.11,12 The topic is important in understanding frozen aqueous systems, which is beyond the scope of this study. No lower temperature real glass transition (Tg) was observed in the thermograms we obtained.Fig. 1 shows the thermograms of frozen solutions containing polyelectrolytes and non-ionic polymers. Some thermograms showed an apparent single transition, while others showed a relatively gradual shift. Combinations of polymers whose single-solute Tg′s are distant clearly demonstrated their varying miscibilities. Frozen solutions containing dextran and polyelectrolytes (PAANa, DEAE-dextran or dextran sulfate) showed large single transitions (−19.7, −21.3, −14.8 °C) between their single-solute Tg′s. This indicates that the polymer combinations are in an amorphous mixture phase in each frozen solution.
 | ||
| Fig. 1 Thermograms of frozen solutions containing a polyelectrolyte and a non-ionic polymer. Aqueous solutions containing a polyelectrolyte and a non-ionic polymer (5% each) were frozen and scanned from −100 °C at 5 °C min−1. The thermograms represent solutions of (1) PAANa and dextran, (2) PAANa and Ficoll, (3) PAANa and PVP, (4) DEAE-dextran and dextran, (5) DEAE-dextran and Ficoll, (6) DEAE-dextran and PVP, (7) dextran sulfate and dextran, (8) dextran sulfate and Ficoll and (9) dextran sulfate and PVP. The Tg′s are marked with triangles. | ||
The combination of 5% PAANa and Ficoll (−34.0 and −25.1 °C) or PAANa and PVP (−34.5 and −26.0 °C) showed two small transitions in gradually inclined thermograms. The two Tg′s were more apparent in derivative thermograms (data not shown). The two Tg′s at temperatures close to their single-solute Tg′s indicated separation into PAANa-rich and Ficoll-rich (or PVP-rich) phases in a frozen solution. These transitions were also observed at lower polymer concentrations (1% PAANa and 1% Ficoll data not shown). The phase separation of PAANa and PVP in a frozen solution agrees with the poor miscibility of the polymers to form an aqueous two-layer solution at room temperature.13 The high concentration of the polymers (above 9% PAANa 185000 and PVP 210000) required for the two-layer formation13 emphasizes the contribution of freeze-concentration on the phase separation observed in the frozen solutions.
The combination of polymers with close single-polymer transition temperatures gave seemingly single transitions (DEAE-dextran and Ficoll, −25.0 °C: DEAE-dextran and PVP, −23.1 °C; dextran sulfate and PVP, −21.0 °C, dextran sulfate and Ficoll −22.3 °C). However, the possibility of the occurrence of either an actual single transition or the overlapping of two transitions makes it difficult to determine the polymer miscibility from these thermograms.
(2) Effect of co-solutes on the miscibility of frozen polymer solutions
Some co-solutes have a large effect on the phase diagrams of aqueous polyelectrolyte–non-ionic polymer systems at ambient temperature.7,8 We studied the effect of low- molecular-weight co-solutes on the miscibility of polymers in frozen solutions using thermal analysis. First, we examined salt-induced Tg′ changes to single-polymer frozen solutions. Fig. 2 shows the effect of NaCl and NaSCN on the Tg′ of some single-polymer frozen solutions. The Tg′ of frozen polymer solutions decreased almost linearly in the presence of up to 200 mM salts. The dextran and DEAE-dextran Tg′s were parallel when they had the same salt concentrations. The transition was broad and unclear in 5% dextran sulfate with 200 mM NaCl. NaCl also lowered the Tg′ of other polymers employed in this study (data not shown). The low Tg′ of the salts should lower that of polymer–salt amorphous mixtures.1,18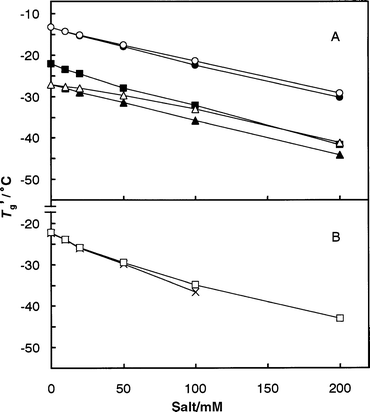 | ||
| Fig. 2 Effect of salts on Tg′s of single-polymer aqueous frozen solutions. Aliquots (10 μl) of aqueous solutions containing 5% w/w polymer and various concentrations of NaCl or NaSCN were scanned from −100 °C at 5 °C min−1. The transition temperatures were obtained from the peaks in derivative thermograms (n=2). ○, dextran+NaSCN; ●, dextran+NaCl; ■, Ficoll+NaCl; △, DEAE-dextran+NaSCN; ▲, DEAE-dextran+NaCl; □, PVP+NaCl; X, dextran sulfate+NaCl. | ||
Fig. 3 shows derivative thermograms of frozen solutions containing 5% PAANa, 5% dextran and various concentrations of NaCl. As noted above, the combination of PAANa and dextran showed a single Tg′ at −19.7 °C. The addition of NaCl shifted the single Tg′ to a lower temperature, indicating that the polymer combination remained mixed in the freeze-concentrated phase in the presence of NaCl. A similar NaCl-induced shift in the single Tg′ was observed in the dextran sulfate–dextran system (data not shown).
 | ||
| Fig. 3 Derivative thermograms of frozen solutions containing PAANa, dextran and NaCl. Aliquots (10 μl) of aqueous solutions containing 5% w/w PAANa, 5% w/w dextran and various concentrations of NaCl were scanned from −100 °C at 5 °C min−1. | ||
Fig. 4 and 5 show derivative thermograms of aqueous frozen solutions containing dextran sulfate, Ficoll (or PVP) and NaCl. The miscibility of dextran sulfate and Ficoll (or PVP) in the absence of NaCl is not clear because of the close single-solute Tg′s and seemingly single Tg′ of the polymer combination. The addition of NaCl induced similar changes in thermograms of these polymer combinations. The Tg′ peak in the derivative thermograms broadened and split in the presence of NaCl. Two transitions were observed in the presence of 50 or 100 mM NaCl, while the further addition of NaCl made the higher temperature Tg′ peak broad and unclear. The two Tg′s indicate that the polymer combination separates into different phases in a frozen solution, at least in the presence of NaCl. Dextran sulfate and various non-ionic polymers (e.g. PVP) form aqueous two-layer solutions in the presence of NaCl,7,8 indicating the unfavorable interaction of the polymers.
 | ||
| Fig. 4 Derivative thermograms of frozen solutions containing dextran sulfate, Ficoll and NaCl. Aliquots (10 μl) of aqueous solutions containing 5% w/w dextran sulfate, 5% (w/w Ficoll and various concentrations of NaCl were scanned from −100 °C at 5 °C min−1. | ||
 | ||
| Fig. 5 Derivative thermograms of frozen solutions containing dextran sulfate, PVP and NaCl. Aliquots (10 μl) of aqueous solutions containing 5% w/w dextran sulfate, 5% w/w PVP and various concentrations of NaCl were scanned from −100 °C at 5 °C min−1. | ||
The difference between the two Tg′s increased when the salt concentrations in the polymer systems were increased. An uneven distribution of NaCl to Ficoll-rich (or PVP-rich) and dextran sulfate-rich phases should explain the difference in the transition temperatures. While NaCl lowers the Tg′s of the single-polymer solutions to a similar extent (Fig. 2), a larger distribution of NaCl to one of the separated phases should lower the Tg′ to a larger degree. Salts are distributed differently in each phase of aqueous two-layer solutions, depending on the interaction between the salt and the polymers.19,20 Thermal analysis using combinations of different polymer concentrations (2 and 5%) suggested the predominant polymer in each phase. Frozen solution containing 5% Ficoll, 2% dextran sulfate and 50 mM NaCl showed a relatively evident lower temperature Tg′ transition (data not shown). The combination of the opposite polymer concentrations (2% Ficoll, 5% dextran sulfate and 50 mM NaCl) made the higher temperature Tg′ transition more apparent. This indicated that NaCl is distributed largely in the Ficoll-rich (or PVP-rich) phase to present a lower Tg′.
Fig. 6 shows the effect of NaCl on the derivative thermograms of frozen solutions containing 5% w/w DEAE-dextran and dextran. The single Tg′ of the mixture solution at −21.3 °C indicates good polymer miscibility in the frozen solution in the absence of co-solutes. After the transition became unclear at 10 mM NaCl, two Tg′s appeared at −26.4 and −16.6 °C at 20 mM NaCl. These Tg′s were close to the single-polymer DEAE-dextran and dextran Tg′s (−27.1 and −13.3 °C, respectively). They should represent a DEAE-dextran-rich lower temperature Tg′ phase and a dextran-rich higher temperature Tg′ phase in the frozen solution. Both of the phases should contain NaCl to some extent. The addition of 20–500 mM NaCl lowered the two Tg′s, suggesting the dis tribution of NaCl to both of the separated phases. The higher temperature Tg′ phase broadened and became obscure in the presence of a high concentration (500 mM) of NaCl. The two Tg′s also appeared with the addition of 10 mM Na2SO4 , 20 mM NaSCN, 20 mM sodium phosphate buffer (pH 7.0) or 50 mM tris(hydroxymethyl)aminomethane–HCl buffer (pH 7.4) (data not shown).
 | ||
| Fig. 6 Derivative thermograms of frozen solutions containing DEAE-dextran, dextran and NaCl. Aliquots (10 μl) of aqueous solutions containing 5% w/w DEAE-dextran, 5% w/w dextran and various concentrations of NaCl were scanned from −100 °C at 5 °C min−1. | ||
Various salts induced the phase separation of frozen DEAE-dextran–dextran solutions. The effect of salts should be characteristic of the polyelectrolyte and non-ionic polymer combination. The aqueous solution of many polyelectrolyte–non–ionic polymer combinations forms two-layer solutions only in the presence of salts. It is possible that the salt-induced phase separation in frozen solutions occurs by the same mechanism that causes salt-induced aqueous two-layer separation.
Interaction between hydrated polymer components determines the miscibility of non-ionic polymers in aqueous solutions.8 In addition, the electrostatic effect contributes to the miscibility of polyelectrolytes and non-ionic polymers. Salts in the polymer solutions should conceal the electrostatic effect that favors the molecular interaction and makes the polyelectrolyte–nonionic polymer phase behavior close to that of ‘‘base polymer’’–non-ionic polymer systems. The molecular interaction between the ‘‘base polymer’’ and the non-ionic polymer should vary depending on their properties. The combination of DEAE-dextran and dextran is miscible in the absence of co-solutes, while the unfavorable interactions between the ‘‘base DEAE-dextran ’’ and dextran should make the polymer combination immiscible in frozen solutions in the presence of salts. In contrast, some combinations of polyelectrolytes and non-ionic polymers (e.g., PAANa and dextran) are miscible even the electrostatic effect is concealed by salts.
Fig. 7 shows the effect of NaSCN on the frozen solution containing 5% DEAE-dextran and dextran. As has been observed with various salts, a low concentration (20 mM) of NaSCN induced the phase separation of the polymers. The two Tg′s appearing at 20 mM NaSCN grew closer by further addition of NaSCN, and they merged at 300 mM NaSCN. The merged Tg′ shifted to lower temperatures at 400 mM NaSCN. The mixing of the two phases, not the overlapping of the independent phase transitions, should merge the Tg′s. The NaSCN-induced Tg′ change of the polymer combination was complex. Whereas the addition of low-concentration (20–200 mM) NaSCN did not alter the lower temperature Tg′ (DEAE-dextran-rich phase) of the frozen solution, a higher concentration (400 mM) of NaSCN shifted the merged Tg′.
 | ||
| Fig. 7 Derivative thermograms of frozen solutions containing DEAE-dextran, dextran and NaSCN. Aliquots (10 μl) of aqueous solutions containing 5% w/w DEAE-dextran, 5% w/w dextran and various concentrations of NaSCN were scanned from −100 °C at 5 °C min−1. | ||
Salts alter the miscibility of non-ionic polymers in aqueous solution and frozen solution, depending on their position in Hofmeister's lyotropic series.21,22 Salting-in salts (e.g., NaSCN, NaI) raise the binodal curve of aqueous two-layer solutions containing two non-ionic polymers by changing the hydration state of the polymers.8,23,24 They also improve the miscibility of non-ionic polymers in frozen solutions. The addition of salting-in salts (e.g., NaSCN, NaI) or intermediate salts (e.g., NaCl) merges the Tg′s of PVP and dextran combinations in frozen solutions,4 while the polymer miscibility decreases in the presence of salting-out salts. The effect of a high concentration of NaSCN, a typical salting-in salt, on the merging of the two Tg′s of DEAE-dextran and dextran should result in improved polymer miscibility in the frozen solution.
The salt-induced phase separation and phase merging were also observed in lower concentration frozen polymer solutions (1% DEAE-dextran and 1% dextran; data not shown). The lower concentrations of salts required for phase separation (10 mM for NaCl or NaSCN) and phase merging (50 mM NaSCN) of the polymer combination suggested that the relative concentration is important in determining the solute miscibility in frozen solutions.
Some mono- and disaccharides are miscible with many polymers in aqueous frozen solutions.2,4 We studied the effect of sucrose on the miscibility of the polyelectrolyte and non-ionic polymer combination in frozen solution. The single Tg′ of the DEAE-dextran–dextran combination shifted to a lower temperature in the presence of sucrose, indicating that the solutes remain in a single phase in the frozen solutions (Fig. 8). The low transition temperature of sucrose (−37.3 °C in 5% solution) should lower the Tg′ of the mixture. Although the effect of sucrose on the salt-induced phase separation of polyelectrolytes and non-ionic polymers in a frozen solution is an intriguing area to study, thermal analysis did not provide clear information about the solute miscibility. For example, a frozen solution containing 5% DEAE-dextran, 5% dextran, 5% sucrose and 20 mM NaCl showed a relatively broad single transition with a peak at −30.2 °C (data not shown). Further study is required for determining the miscibility of components in these systems.
 | ||
| Fig. 8 Derivative thermograms of frozen solutions containing DEAE-dextran, dextran and sucrose. Aliquots (10 μl) of aqueous solutions containing 5% w/w DEAE-dextran, 5% w/w dextran and various concentrations of sucrose were scanned from −100 °C at 5 °C min−1. | ||
Conclusion
Combinations of polyelectrolytes and non-ionic polymers showed varied miscibility in frozen solutions. While some polymers (e.g., PAANa–dextran, DEAE-dextran–dextran) were concentrated in a mixture phase, others (e.g., PAANa and Ficoll) were separated into different phases. The basic mechanism of the freeze-induced phase separation, namely freeze-concentration of polymers that interacted unfavorably, should also apply in the polyelectrolyte–non-ionic polymer system.The effect of salts varied, depending on the polymer combinations. Low concentrations of salts diminish the electrostatic effects favorable for polymer interactions. This effect is most apparent in polymer combinations whose miscibility changes to immiscibility in the presence of low concentrations of polymers (e.g., DEAE-dextran and dextran). The addition of a salt also affected polymer miscibilities by altering the polymer's hydration state. Higher concentration of a salting-in salt (NaSCN) made the DEAE-dextran and dextran combination miscible. The kind of polymers and co-solutes and their relative concentration should determine the solute miscibility in polyelectrolyte–non-ionic polymer frozen solutions. Controlling the solute miscibility through formulation and process design will be important in producing freeze-dried biopharmaceuticals.25,26
References
- L. M. Her, M. Deras and S. L. Nail, Pharm. Res., 1995, 12, 768 CrossRef CAS.
- K. Izutsu, S. Yoshioka, S. Kojima, T. W. Randolph and J. F. Carpenter, Pharm. Res., 1996, 13, 1393 CrossRef CAS.
- T. W. Randolph, J. Pharm. Sci., 1997, 86, 1198 CrossRef CAS.
- K. Izutsu, M. C. Heller, T. W. Randolph and J. F. Carpenter, J. Chem. Soc., Faraday Trans., 1998, 94, 411 RSC.
- A. P. MacKenzie, Dev. Biol. Stand., 1977, 36, 51 Search PubMed.
- P.-Å. Albertsson, Biochim. Biophys. Acta, 1958, 27, 378 CAS.
- P.-Å. Albertsson, Partition of Cell Particles and Macromolecules, Wiley, New York, 1986. Search PubMed.
- B. Y. Zaslavsky, Aqueous Two-Phase Partitioning, Marcel Dekker, New York, 1995. Search PubMed.
- E. Shalaev and F. Franks, Cryobiology, 1996, 33, 14 CrossRef.
- A. P. Mackenzie, Philos. Trans. R. Soc. London, Ser. B, 1971, 278, 167 Search PubMed.
- B. S. Chang and C. Randall, Cryobiology, 1992, 29, 632 CrossRef CAS.
- E. Y. Shalaev and F. Franks, J. Chem. Soc., Faraday Trans., 1995, 91, 1511 RSC.
- M. B. Perrau, I. Illiopoulos and R. Audebert, Polymer, 1989, 30, 2112 CrossRef CAS.
- K. Izutsu, S. Yoshsioka and T. Terao, Pharm. Res., 1993, 10, 1232 CrossRef CAS.
- M. C. Heller, J. F. Carpenter and T. W. Randolph, J. Pharm. Sci., 1996, 85, 1358 CrossRef CAS.
- J. F. Carpenter and J. H. Crowe, Biochemistry, 1989, 28, 3916 CrossRef CAS.
- L.-M. Her and S. L. Nail, Pharm. Res., 1994, 11, 54 CrossRef CAS.
- M. Jochem and C. H. Körber, Cryobiology, 1987, 24, 513 CrossRef CAS.
- G. Johansson, Biochim. Biophys. Acta, 1970, 221, 387 CAS.
- B. Y. Zaslavski, L. M. Miheeva and Y. P. Aleschko-Ozhevskii, J. Chromatogr., 1988, 439, 267 CrossRef.
- F. Hofmeister, Arch. Exp. Pathol. Pharmakol., 1888, 24, 247 Search PubMed.
- S. Saito, J. Polym. Sci., Polym. Chem. Ed., 1969, 7, 1789 CAS.
- D. E. Brooke, K. A. Sharp, S. Bamberger, C. H. Tamblyn, G. V. F. Seaman and H. Walter, J. Colloid Interface Sci., 1984, 102, 1 CrossRef.
- B. Y. Zaslavski, T. O. Bagirov, A. A. Borovskaya, G. Z. Gasanova, N. D. Gulaeva, V. Y. Levin, A. A. Masimov, A. U. Mahmudov, L. M. Miheeva, N. N. Osipov and S. V. Rogozhin, Colloid Polym. Sci., 1986, 264, 1066.
- M. J. Pikal, BioPharm, 1990, 3, 26 Search PubMed.
- M. C. Heller, J. F. Carpenter and T. W. Randolph, Biotechnol. Bioeng., 1999, 63, 164 CrossRef.
| This journal is © the Owner Societies 2000 |
