Molecular capsule constructed by multiple hydrogen bonds: self-assembly of cavitand tetracarboxylic acid with 2-aminopyrimidine
Kenji
Kobayashi
*a,
Toshiaki
Shirasaka
a,
Kentaro
Yamaguchi
b,
Shigeru
Sakamoto
b,
Ernst
Horn
a and
Naomichi
Furukawa
a
aDepartment of Chemistry and Tsukuba Advanced Research Alliance
Center, University of Tsukuba, Tsukuba, Ibaraki, 305-8571, Japan.. E-mail: kenjinor@staff.chem.tsukuba.ac.jp
bChemical Analysis Center, Chiba University, Inage-ku, Chiba, 263-8522, Japan
cDepartment of Chemistry, Rikkyo University, Nishi-Ikebukuro, Toshima-ku, Tokyo, 171-8501, Japan
First published on 7th January 2000
Abstract
Two molecules of cavitand tetracarboxylic acid and four molecules of 2-aminopyrimidine assemble into a capsule via 16 hydrogen bonds, as shown by 1H NMR titration and X-ray crystallographic analysis; in the solid state, two molecules of nitrobenzene are encapsulated in the capsule.
Error correction through thermodynamic equilibration, minimization of synthetic effort by use of modular subunits and control of assembly processes through subunit design are characteristic of supramolecular approaches to self-assembly.1,2 Carcerands, in which two calix[4]resorcinarene cavitands are held together by four covalent linkages, have been synthesized and well-characterized by Cram and others, and have attracted considerable attention from the viewpoint of stabilization of reactive intermediates and microvesicles for drug delivery by confinement of guest molecules inside the capsules away from bulk phases.3 Recently, the field of molecular capsules has advanced to a stage where self-assembly through noncovalent interactions such as hydrogen bonds and metal coordination has proved to be a reliable tool.4,5 Based on hydrogen bonds, homodimers of functionalized cavitands in solution6,7 and multicomponent assemblies of calix[4]resorcinarenes with solvent water or alcohol molecules as linkers in the solid state8,9 have been reported. Metal-bridged assemblies of functionalized cavitands have also proved to be a viable alternative.10 We have chosen the cavitand tetracarboxylic acid 1 as a concave subunit11 and 2-aminopyrimidine (2-AP) as a hydrogen-bonded linker subunit. Our strategy for capsule formation is based on the combination of carboxylic acids with 2-AP to form a 2∶1 hydrogen-bonded complex in the solid state.12 Herein we report the construction of capsule 2 from six components via 16 hydrogen bonds: two molecules of 1 are indirectly held together in a rim-to-rim fashion by hydrogen bonding bridges involving four molecules of 2-AP (Scheme 1).
 | ||
| Scheme 1 | ||
The cavitand 1 alone has a low solubility in CHCl3 at room temperature {<5 mM for 1a [R = (CH2)6CH3] and <1 mM for 1b [R = (CH2)2Ph]}. On the other hand, in the presence of 2 equiv. of 2-AP, 1 becomes very soluble in CHCl3 (>100 mM). The titration of a suspension of 1a in CDCl3 at 25 °C with aliquots of 2-AP was monitored by 1H NMR spectroscopy. Fig. 1 shows the chemical shift changes for the NH2 protons of 2-AP and the inward and outward methylene protons at the rim of 1a as a function of 2-AP/1a.† Below the 2∶1 ratio of 2-AP/1a, the NH2 protons of 2-AP apparently give a saturated downfield shift (Δδobs = 2.95 ppm), independent of 2-AP/1a, while the methylene protons of 1a are increasingly shifted downfield upon addition of 2-AP. At the 2∶1 stoichiometry of 2-AP/1a, the mixture becomes completely homogeneous.† Beyond the 2∶1 ratio, the former chemical shift changes decrease due to the average of signals between the free and complex 2-AP, whereas the latter ones remain unchanged. The fact that both titration curves have an inflection point at the 2∶1 stoichiometry of 2-AP/1a indicates that an n∶2n complex of 1a with 2-AP is formed via hydrogen bonds and that this formation may be a cooperative process. The cavitand 1a and 2-AP possess four and two hydrogen bonding sites, respectively. In principle, 1∶2 and/or 1∶4 complexes of 1a with 2-AP may be conceivable. In such complexes, however, half of the hydrogen bonding sites still remain intact. The titration data obtained here suggests the formation of a capsule 2a in solution from two molecules of 1a and four molecules of 2-AP (Scheme 1).‡ The capsule 2a in CDCl3 was disrupted upon addition of DMSO-d6 as a cosolvent.
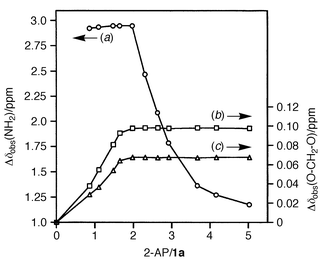 | ||
| Fig. 1 Chemical shift changes for (a) the NH2 protons of 2-AP and (b) the inward and (c) outward methylene protons at the rim of 1a as a function of 2-AP/1a in CDCl3 at 25 °C. | ||
The stability of 2a was evaluated by the dilution method in CDCl3 at 25 °C using 1H NMR, where the ratio of 2-AP/1a is maintained at 2∶1. The chemical shift changes for the NH2 protons of 2-AP decreased at less than 10 mM of 2-AP. The association constant for the formation of 2a calculated from a nonlinear curve fitting was estimated to be Ka = 3.7 × 1019 M−5 in CDCl3 at 25 °C with Δδsat = 2.96 ppm and a correlation coefficient r = 0.99.§ This corresponds to an average ΔG° = −1.6 kcal mol−1 per hydrogen bond.13
Recrystallization of a mixture of 1 and 2-AP from various
solvents such as p-xylene, anisole–CHCl3 and
nitrobenzene–CHCl3 gave co-crystals composed of
1∶2-AP = 1∶2 without exception. The IR spectra showed
a shift (10 cm−1) to lower wavenumbers of the
νC![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O for 1 upon adduct formation. The
O–H stretching bands appeared at 2500 and 1900 cm−1,
which are characteristic of a carboxylic acid hydrogen bonded to an
aromatic ring nitrogen.12 When pyridine was used in place of
2-AP, co-crystals of 1∶pyridine = 1∶4 were obtained.
These results support the formation of 2 from 1 and 2-AP
in the solid state.
O for 1 upon adduct formation. The
O–H stretching bands appeared at 2500 and 1900 cm−1,
which are characteristic of a carboxylic acid hydrogen bonded to an
aromatic ring nitrogen.12 When pyridine was used in place of
2-AP, co-crystals of 1∶pyridine = 1∶4 were obtained.
These results support the formation of 2 from 1 and 2-AP
in the solid state.
Single crystals suitable for X-ray diffraction analysis were grown by allowing a hot solution of 1b and2-AP in nitrobenzene to slowly cool to room temperature.¶ As shown in Fig. 2, the molecular structure after symmetry operations unambiguously reveals the capsule 2b in which two molecules of the hemispherical cavitand 1b associate indirectly in a rim-to-rim fashion by hydrogen bonding bridges involving four molecules of 2-AP located on an equatorial position. The NH2 protons and aromatic ring nitrogens of 2-AP form hydrogen bonds with the acid carbonyl oxygens and OH protons of 1b, respectively. The hydrogen bonding distances of N(H)⋯O are 2.866(7), 2.841(7), 2.897(7) and 2.872(7) Å, and those of N⋯(H)O are 2.770(7), 2.604(7), 2.711(6) and 2.718(6) Å. Thus, the capsule 2b is constructed via 16 hydrogen bonds between two molecules of 1b and four molecules of 2-AP. The dihedral angles between the carboxylic acid groups and the resorcinol rings of 1b are almost perpendicular (83.5, 85.7, 105.9 and 80.2°), probably due to the electronic repulsion between the oxygen atoms of their moieties. This orientation plays an important role in the formation of the capsule. The capsule 2b features dimensions of ca. 9 × 15 Å. Two molecules of nitrobenzene are encapsulated in the cavity of 2b in an antiparallel fashion with an inter-ring distance of ca. 3.3 Å. They are oriented with the nitro groups towards the equatorial windows of 2b.
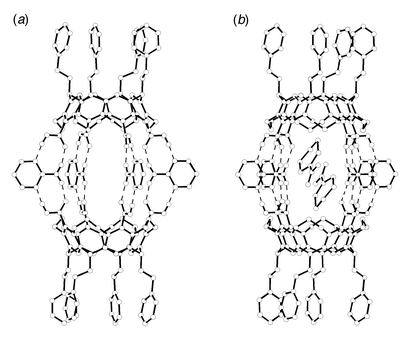 | ||
| Fig. 2 Molecular structure of capsule 2b (a) without and (b) with encapsulated nitrobenzenes. Nitrobenzenes which are not located inside the cavity and hydrogen atoms except for NH2 protons of 2-AP and acid protons of 1b are omitted for clarity. Dashed lines represent intermolecular hydrogen bonds. | ||
In summary, we have demonstrated the capsule 2, composed of two molecules of the cavitand tetracarboxylic acid 1 and four molecules of 2-aminopyrimidine, as a hydrogen-bonded linker. Exploration of linkers such as bipyridine derivatives and transition metals in order to control the cavity size of the capsules is currently underway in our laboratory.
References
- J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995. Search PubMed.
- G. M. Whitesides, E. E. Simanek, J. P. Mathias, C. T. Seto, D. N. Chin, M. Mammen and D. M. Gordon, Acc. Chem. Res., 1995, 28, 37 CrossRef CAS; D. S. Lawrence, T. Jiang and M. Levett, Chem. Rev., 1995, 95, 2229 CrossRef CAS.
- D. J. Cram and J. M. Cram, Container Molecules and Their Guests, Royal Society of Chemistry, Cambridge, 1994; Search PubMed; A. Jasat and J. C. Sherman, Chem. Rev., 1999, 99, 931 Search PubMed.
- For molecular capsules via hydrogen bonds, see: M. M. Conn and J. Rebek Jr.,Chem. Rev., 1997, 97, 1647; Search PubMed; J. de Mendoza, Chem. Eur. J., 1998, 4, 1373 Search PubMed; J. Rebek Jr., Acc. Chem. Res., 1999, 32, 278 CrossRef CAS.
- For molecular capsules via metal coordination, see: M. Fujita, D. Oguro, M. Miyazawa, H. Oka, K. Yamaguchi and K. Ogura, Nature, 1995, 378, 469; Search PubMed; B. Olenyuk, A. Fechtenkötter and P. J. Stang, J. Chem. Soc., Dalton Trans., 1998, 1707 Search PubMed.
- R. G. Chapman and J. C. Sherman, J. Am. Chem. Soc., 1995, 117, 9081 CrossRef CAS; R. G. Chapman, G. Olovsson, J. Trotter and J. C. Sherman, J. Am. Chem. Soc., 1998, 120, 6252 CrossRef CAS.
- T. Heinz, D. M. Rudkevich and J. Rebek Jr., Nature, 1998, 394, 764 CrossRef CAS.
- L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS.
- K. N. Rose, L. J. Barbour, G. W. Orr and J. L. Atwood, Chem. Commun., 1998, 407 RSC; K. Murayama and K. Aoki, Chem. Commun., 1998, 607 RSC.
- P. Jacopozzi and E. Dalcanale, Angew. Chem., Int. Ed. Engl., 1997, 36, 613 CrossRef CAS; O. D. Fox, N. K. Dalley and R. G. Harrison, J. Am. Chem. Soc., 1998, 120, 7111 CrossRef CAS.
- H.-J. Choi, D. Bühring, M. L. C. Quan, C. B. Knobler and D. J. Cram, J. Chem. Soc., Chem. Commun., 1992, 1733 RSC.
- M. C. Etter and D. A. Adsmond, J. Chem. Soc., Chem. Commun., 1990, 589 RSC.
- C. T. Seto and G. M. Whitesides, J. Am. Chem. Soc., 1993, 115, 1330 CrossRef CAS.
Footnotes | |||
| † The concentration of 1a is 20 mM if completely soluble in CDCl3. The equilibrium is reached immediately. At the 2∶1 stoichiometry of 2-AP/1a, the signal of the acid protons of 1a was observed at δ 16.00 at −30 °C. Irradiation of the C(4,6) hydrogens of 2-AP showed a 3% NOE at the acid protons of 1a at −30 °C. | |||
| ‡ No evidence for the encapsulation of guests into 2a in CDCl3 is available, probably due to favorable accommodation of CDCl3 as a bulk phase in 2a. | |||
§ A constant ratio [2-AP]∶[1a] = 2∶1, and
the assumption that free 1a, free 2-AP and capsule 2a are
the only components present, give eqn. (1),
| |||
¶ Crystal data for
1b·2(2-AP)·6(PhNO2):
C112H96N12O28, M =
2058.05, crystal size 0.52 × 0.45 × 0.40 mm, triclinic, space
group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 17.79(2), b = 19.78(1),
c = 15.047(5) Å, α = 107.32(4), β = 96.18(3),
γ = 79.05(5)°, U = 4955(6) Å3,
Z = 2, Dc = 1.379 g
cm−3, μ(Mo-Kα) = 1.00 cm−1,
2θmax = 50.2°. Intensity data (11593) were measured
on a Rigaku RAXIS-II diffractometer at 120 K. The final least-squares
refinement based on F for 8815 unique reflections [with I
> 3.0ς(I)] and 1396 parameters converged with R
= 0.110 and Rw = 0.124. CCDC
182/1489. See http://www.rsc.org/suppdata/cc/a9/a908315d/ for
crystallographic data in .cif format. , a = 17.79(2), b = 19.78(1),
c = 15.047(5) Å, α = 107.32(4), β = 96.18(3),
γ = 79.05(5)°, U = 4955(6) Å3,
Z = 2, Dc = 1.379 g
cm−3, μ(Mo-Kα) = 1.00 cm−1,
2θmax = 50.2°. Intensity data (11593) were measured
on a Rigaku RAXIS-II diffractometer at 120 K. The final least-squares
refinement based on F for 8815 unique reflections [with I
> 3.0ς(I)] and 1396 parameters converged with R
= 0.110 and Rw = 0.124. CCDC
182/1489. See http://www.rsc.org/suppdata/cc/a9/a908315d/ for
crystallographic data in .cif format. |
| This journal is © The Royal Society of Chemistry 2000 |
