Kinetic resolution of amines by acylation using 3-diacylaminoquinazolin-4(3H)-ones
Abdullah G.
Al-Sehemi
,
Robert S.
Atkinson
,
John
Fawcett
and
David R.
Russell
Department of Chemistry, Leicester University, Leicester, UK LE1 7RH. E-mail: vow@le.ac.uk
First published on 24th December 1999
Abstract
Four diastereoisomeric 3-diacylaminoquinazolinones 8a–d have been separated and identified by X-ray structure determinations on three of them: their stoichiometric reactions with α-phenylethylamine and with 2-methylpiperidine (2 equiv. of amine) gave the corresponding N-(2-acetoxypropanoyl)amine and unreacted amine in high diastereomeric/enantiomeric excess.
3-Diacylaminoquinazolinones (DAQs), e.g.1, are highly selective acylating agents for primary amines in the presence of secondary amines and for the less hindered of two secondary amines.1 Recently the N,N-diacetamide 2 has been claimed to be even more chemoselective than DAQ 1 as an acetylating agent: a 1∶1 mixture of pyrrolidine and piperidine (ΔpKa = 0.01) reacts with 2 to give a 15∶1 ratio of the corresponding N-acetylated amines.2
The DAQs 3, 4 and 5 were available to us by acetylation of the corresponding 3-aminoquinazolinones3 and, when reacted with a mixture of pyrrolidine and piperidine (1 equiv. each) at 0 °C in CH2Cl2 gave the ratios of the corresponding amides shown in Scheme 1. Unexpectedly, the chemoselectivity increases as the substituent R on the chiral centre decreases in size from But → Me with a 20∶1 ratio for methyl.†
 | ||
| Scheme 1 | ||
Since DAQs 3, 4 and 5 are enantiopure we were particularly interested in a greater potential advantage which their use offered over that of e.g.2, namely as enantioselective acetylating agents.4 However, reaction of DAQ 3 (1 equiv.) with racemic 2-methylpiperidine (2 equiv.) gave a sample of recovered amine having zero optical rotation and hence there is no kinetic resolution in this acetylation.
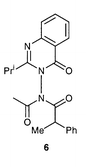
For 3-aminoquinazolinones N,N-disubstituted with different acyl groups, we have previously shown that the N–N bond is a chiral axis: in DAQ 6 the presence of two chiral elements gives rise to diastereoisomers with a barrier ΔG# = 121 kJ mol−1 for their interconversion by rotation around the N–N bond.4
Successive acylation of enantiopure 3-aminoquinazolinone 73 with (S)-2-acetoxypropanoyl chloride and isobutanoyl chloride (Scheme 2) gave four DAQ diastereoisomers 8a–d in order of their elution on chromatography (see below). The major diastereoisomer 8b was separated by crystallisation (35%) after flash chromatography to remove unreacted mono-acylquinazolinone (MAQ) and pure samples of the other three, 8a (5%), 8c (7%) and 8d (26%), were obtained by separation using a chromatotron.
 | ||
| Scheme 2 | ||
X-Ray crystal structure determinations of DAQs 8b and 8c (Fig. 1) showed them to have the expected (S)-configuration at their α-acetoxypropanoyl chiral centres but different configurations at their N–N chiral axes.‡ Heating (CDCl3, 60 °C, 42 h) interconverted 8b and 8c and gave a 5∶4 ratio respectively at equilibrium, presumably as a result of rotation around the N–N bond: no interconversion with 8a or 8d occurred.
 | ||
| Fig. 1 Molecular structures of (a) 8b, (b) 8c and (c) 8d showing atom labelling schemes and 30% probability displacement parameters. H atoms at chiral centres are shown with dashed bonds, all other H atoms are omitted for clarity. | ||
Diastereoisomers 8a and 8d arise from epimerisation at the α-acetoxypropanoyl centre as shown by the (R)-configuration in the X-ray crystal structure of 8d (Fig. 1).‡ Interconversion of 8a and 8d by heating (CDCl3, 60 °C, 13 h) gave a 6∶1 ratio at equilibrium, respectively: no interconversion with 8b and 8c occurred in this equilibrium.
Although in their crystal structures DAQ 8b–d all have the usual4exo/endo conformation for their imides, 8b and 8c have their α-acetoxypropanoyl groups endo and the isobutanoyl group exo whereas 8d has the positions of these two groups reversed.
We find that these DAQs 8a–d are enantioselective acylating agents for racemic amines. Thus DAQ 8c (1 equiv.) reacts with α-phenylethylamine (2 equiv.) in CH2Cl2 at 5 °C to give (2R,2′S)-N-(-2-acetoxypropanoyl)-α- phenylethylamine [77% based on (R)-α-phenylethylamine, 88% de] by NMR comparison with authentic samples of both diastereoisomers: the recovered S enantiomer of the amine [90% based on (S)-α-phenylethylamine] was of 91% ee from its specific rotation (Scheme 3).
 | ||
| Scheme 3 | ||
Reaction of DAQ 8c (1 equiv.) with racemic 2-methylpiperidine 10 (2 equiv.) in CH2Cl2 at 5 °C for 24 h gave, after chromatography, monoacylquinazolinone (MAQ) 11 (77%) and (2S,2′S)-1-(2′-acetoxypropanoyl)-2-methylpip eridine 9a (79%) (Scheme 3) whose diastereopurity (de) as measured by NMR spectroscopy was 95%. The unreacted 2-methylpiperidine enantiomer was recovered from the crude reaction mixture by extraction with HCl (2 M) and derivatised by reaction with (S)-2-acetoxypropanoyl chloride to give 9b (81%) of 89% de: at 400 MHz and 50 °C the OCOCH3 signals of diastereoisomers 9a and 9b are well separated.§
Since DAQ 8b was recovered unchanged when re-subjected to the conditions of the second acylation in Scheme 2, it appears that epimerisation at the 2-acetoxypropanoyl chiral centre occurs in the second acylation step.¶ An alternative, more stereoselective route to these DAQs was to carry out the second acylation using the lithium salt of the MAQ. Thus reaction of the lithium salt of MAQ 11 with (S)-2-acetoxypropanoyl chloride gave DAQ 8c (71%) and 8a (8%) (based on recovered starting material), i.e. with less epimerisation than previously.
Kinetic resolution is usually carried out in the presence of excess of the substrate to maximise enantiopurity in its derivatised enantiomer. Even under the most testing conditions of stoichiometry as in Scheme 3, DAQ 8c delivers both derivatised and unreacted enantiomers of the substrate with high enantioselectivity.
Acknowledgements
We thank the Ministry of Education, Saudi Arabia for funding (to A. G. Al-S).References
- R. S. Atkinson, E. Barker and M. J. Sutcliffe, Chem. Commun., 1996, 105 RSC.
- Y. Murakami, K. Kendo, K. Miki, Y. Akiyama, T. Watanabe and Y. Yokoyama, Tetrahedron Lett., 1997, 38, 3751 CrossRef CAS.
- R. S. Atkinson, A. P. Ayscough, W. T. Gattrell and T. M. Raynham, J. Chem. Soc., Perkin Trans. 1, 1998, 2783 RSC.
- R. S. Atkinson, E. Barker, P. J. Edwards and G. A. Thomson, J. Chem. Soc., Perkin Trans. 1, 1996, 1047 RSC.
Footnotes |
| † This increase may be related to the preference for a defined conformation around the C–OSi bond in the (Q) 2-substituent in 3 but not in 4 or 5 (see R. S. Atkinson, M. P. Coogan and I. S. T. Lochrie, Tetrahedron Lett., 1996, 37, 5179. |
| ‡ Crystal data for 8b: C27H41N3O6Si, M = 531.72, orthorhombic, space group P212121, a = 10.146(1), b = 13.296(2), c = 22.618(3) Å, V = 3051.4(7) Å3, Z = 4, μ(Mo-Ka) = 0.118 mm−1, 3845 reflections measured, 3639 unique (Rint = 0.013) which were all used in calculations. Final R1 = 0.052 and wR2 = 0.114 (all data). For 8c: C27H41N3O6Si, M = 531.72, orthorhombic, space group P212121, a = 9.391(2), b = 13.554(6), c = 23.908(13) Å, V = 3043(2) Å3, Z = 4, μ(Mo-Ka) = 0.118 mm−1, 3756 reflections measured, 3592 unique (Rint = 0.035) which were all used in calculations. Final R1 = 0.102 and wR2 = 0.309 (all data). For 8d: C27H41N3O6Si, M = 531.72, orthorhombic, space group P212121, a = 12.152(9), b = 13.170(7), c = 18.293(9) Å, V = 2928(3) Å3, Z = 4, μ(Mo-Ka) = 0.118 mm−1, 3666 reflections measured, 3455 unique (Rint = 0.155) which were all used in calculations. Final R1 = 0.044 and wR2 = 0.099 (all data). Data were measured on a Siemens P4 diffractometer at 190 K using graphite monochromated Mo-Ka radiation (λ = 0.7107 Å) using an ω-scan technique. Three standard reflections monitored every 100 scans showed no significant variation in intensity, the reflections were corrected for Lorentz and polarisation effects. The structures were solved by direct methods and refined by full-matrix least-squares on F2. The absolute configurations of the compounds were established by the known configuration at the silyloxy substituted carbon atom. All examined crystals of 8c diffracted weakly; to preserve an observed data to variable ratio of 6∶1 the 13 most closely isotropic atoms were constrained to be isotropic. All hydrogen atoms were included in calculated positions (C–H = 0.96 Å) using a riding model. CCDC 182/1486. See http://www.rsc.org/suppdata/cc/a9/a907970j/ for crystallographic data in .cif format. |
| § A mixture of amide diastereoisomers 9a and 9b was prepared by reaction of (S)-2-acetoxypropanoyl chloride with racemic 2-methylpiperidine. An authentic sample of amide 9b was prepared from (R)-2-methylpiperidine, [α]D (HCl salt) 8.9 (c 2, EtOH), itself prepared from the racemic amine by resolution using (R)-mandelic acid. |
| ¶ Significantly, the MAQ recovered from the reaction in Scheme 2 was also found to be a mixture of two diastereoisomers, epimeric at the 2-acetoxypropanoyl chiral centre. |
| This journal is © The Royal Society of Chemistry 2000 |