29Si and 27Al MAS NMR spectra are affected by alkali metal cluster formation in zeolite LTA
Kelly L. Morana, Peter D. Barkerb, Jennifer E. Readmanb, Peter P. Edwardsb, Ray Dupree*a and Paul A. Anderson*b
aDepartment of Physics, University of Warwick, Coventry, UK CV4 7AL
bSchool of Chemistry, The University of Birmingham, Edgbaston, Birmingham, UK B15 2TT. E-mail: p.a.anderson@bham.ac.uk
First published on UnassignedUnassigned7th January 2000
Abstract
We report the application of 27Al and 29Si MAS NMR to provide a direct probe of alkali metal cluster formation and distribution in the α-cages of zeolite A (LTA).
The reaction of alkali metals with framework aluminosilicates produces a rich variety of inclusion compounds comprising metal clusters and filamentary structures, confined and ordered, within the intracrystalline channels and cavities.1 With zeolite pore dimensions typically ca. 1 nm or less, these may be regarded as the ultimate nanostructured materials, and as such have justifiably attracted considerable experimental and theoretical attention on account of a range of remarkable electronic, magnetic, optical and catalytic properties.2–6 Although the presence in such compounds of low-nuclearity (n = 6) paramagnetic clusters is well documented,7–12 these are present as minority species in all but a few cases.1,2 Pioneering 23Na MAS studies by Nakayama et al.13 have suggested the possible formation of Na53+ and Na−, in zeolites X, Y and A, but in general there is a dearth of information about the diamagnetic species present, in particular higher nuclearity clusters (n > 10) presumed to occupy the larger zeolite cages. Here, we report the first application of 27Al and 29Si MAS NMR to provide a direct probe of cluster formation and distribution in the α-cages of zeolite A.
We prepared a number of deeply coloured compounds M′x/M-A (where A denotes the LTA framework)14 by exposing alkali metal (M) ion-exchanged and dehydrated zeolite samples to a measured amount of alkali metal (M′) vapour in sealed, evacuated quartz tubes.15,16 The products, which were virtually black, were subsequently handled under dry Ar. Unusual MAS NMR17 spectral shifts (Table 1) were observed for several compositions, and for the first time in zeolite A, a number of compounds exhibited two distinct signals (Figs. 1 and 2) for each Td nucleus (Td = 27Al and 29Si), even though the materials were crystallographically single phase.18 When exposed to air the colour centres were gradually quenched, yielding white powders, whose MAS NMR spectra closely resembled those of the ‘empty’ dehydrated hosts, with only one Td signal. Dehydrated zeolite K-A exhibited a single sharp resonance in both the 27Al and 29Si MAS NMR spectra, at the usual shifts for zeolite A (δ ca. 58 and −89, respectively). In metal-loaded ‘black’ K5/K-A, however, sharp Td resonances shifted to lower frequency, δ 52.5 and −100.2, respectively, were observed. The magnitudes of the shifts suggest a structural and/or electronic perturbation of the framework.
| Material | 27Al (line 1) | 27Al (line 2) | 29Si (line 1) | 29Si (line 2) | Ratio 1∶2 |
|---|---|---|---|---|---|
| a 27Al shift measured at 93.8 MHz. For comparison, the 93.8 MHz 27Al shifts of Cs5/K-A are at δ 57.5 and 51.0 | |||||
| Cs5/K-A | 59.3 | 52.2 (2150, total) | −89.7 (540) | −101.9 (420) | 3∶2 |
| Exposed Cs5/K-A | 59.9 (1160) | — | −89.5 (320) | — | — |
| K5/K-A | — | 52.5a | — | −100.2 (400) | — |
| K-A host (dehydrated) | 57.7 (890) | — | −89.3 (490) | — | — |
| Rb7/Rb-A | 83.8 | 67.9 (4320, total) | −64.1 (910) | −86.1 (840) | 3∶1 |
| Exposed Rb7/Rb-A | 61.0 (940) | — | −86.1 (570) | — | — |
| Rb-A host (dehydrated) | 57.8 (700) | — | −89.1 (490) | — | — |
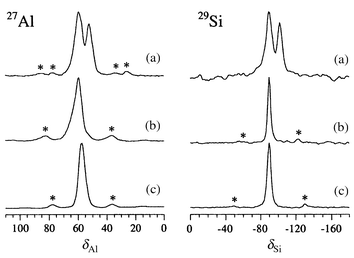 | ||
| Fig. 1 27Al and 29Si MAS NMR spectra of (a) ‘black’ Cs5/K-A, and (b) air-exposed ‘white’ Cs5/K-A and (c) dehydrated ‘empty’ K-A. Asterisks denote positions of spinning sidebands. | ||
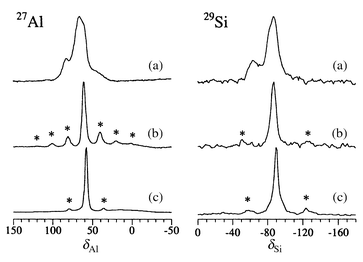 | ||
| Fig. 2 27Al and 29Si MAS NMR spectra of (a) ‘black’ Rb7/Rb-A, (b) air-exposed ‘white’ Rb7/Rb-A and (c) dehydrated ‘empty’ Rb-A. Asterisks denote spinning sidebands. | ||
Topologically, LTA is a cubic array of sodalite cages mutually separated
by double 4-ring (D4R) windows to form a large α-cage. Each
Td atom in the structure belongs to one sodalite cage,
two α-cages and one double 4-ring. In dehydrated K-A, all the
α-cages contain eight K+ cations that are coordinated to
oxygen atoms in the 6-rings and partly balance the negative charge of the
framework. The structure has a pseudo unit cell size (assuming no Si, Al
ordering) of ca. 12.3 Å in space group
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. In K5/K-A, alternate
α-cages contain either eight K+ cations coordinated to
6-rings as in the dehydrated host, or a K124+ cluster
whose partly reduced potassium cations occupy 4-ring sites. This results in
a doubling of the unit cell to 24.6324 Å in space group
Fm
m. In K5/K-A, alternate
α-cages contain either eight K+ cations coordinated to
6-rings as in the dehydrated host, or a K124+ cluster
whose partly reduced potassium cations occupy 4-ring sites. This results in
a doubling of the unit cell to 24.6324 Å in space group
Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.16,19 Owing
to the strict alternation of the cage contents, the structure retains a
single unique crystallographic Td site.16 We suggest that the observed low frequency
Td shifts in K5/K-A may be due to an
increase in the average Td–O–Td
bond angles20 in the cluster-containing
material relative to those in the empty host, which is consistent with an
observed increase in lattice parameter.16,19 It is interesting that there is no paramagnetic
Td shift or broadening of lines observed in this
compound, suggesting that the Td atoms do not interact
appreciably with the paramagnetic clusters known to be present.16,19
m.16,19 Owing
to the strict alternation of the cage contents, the structure retains a
single unique crystallographic Td site.16 We suggest that the observed low frequency
Td shifts in K5/K-A may be due to an
increase in the average Td–O–Td
bond angles20 in the cluster-containing
material relative to those in the empty host, which is consistent with an
observed increase in lattice parameter.16,19 It is interesting that there is no paramagnetic
Td shift or broadening of lines observed in this
compound, suggesting that the Td atoms do not interact
appreciably with the paramagnetic clusters known to be present.16,19
Similar low frequency NMR shifts were observed for Cs5/K-A, but in this case, the deeply coloured material exhibited two sharp resonances for both Td nuclei (Fig. 1). The first one, labelled line 1, is only slightly shifted relative to the dehydrated host and is assigned to Td atoms at the vertices of α-cages that contain only 6-ring cations. Line 2 resembles the Td resonance in K5/K-A, both in shift and signal breadth, and therefore is assigned to Td atoms which are part of one α-cage that contains 6-ring cations and one that contains a ‘reduced’ cluster. As caesium cations are both less likely to occupy the 4-ring site in preference to potassium, and less likely to be found partly reduced, we speculate that K124+ clusters may again be present. Structural studies are under way to confirm this hypothesis.18 The relative intensities of the two lines are consistent with the presence of a cluster in about one fifth of the α-cages. Exposure of ‘black’ Cs5/K-A to air resulted in complete loss of colour and a single Td resonance in both the 27Al and 29Si MAS NMR spectra (Fig. 1).
Two distinct Td resonances were also observed in ‘black’ Rb7/Rb-A and these collapsed to a single signal when the samples were exposed to air (Fig. 2). In this case the lines are significantly broader than in the dehydrated host, which may indicate some site distribution (static disorder) or broadening from nearby paramagnetic clusters. Additionally, both lines are shifted to higher frequency relative to the host. The framework in this fully loaded zeolite is also expanded relative to the empty host,18 therefore there is no straightforward structural explanation for the observed shifts. One plausible explanation is that the framework in this case is directly affected by paramagnetic clusters of guest atoms, similar to the shift trends observed in ‘black’ sodalite.12
We thank the EPSRC for financial support. PAA is a Royal Society University Research Fellow.
References
- P. P. Edwards, P. A. Anderson and J. M. Thomas, Acc. Chem. Res., 1996, 29, 23 and references therein; see also refs. 2–13, 15, 16 and 19. Search PubMed.
- P. P. Edwards, L. J. Woodall, P. A. Anderson, A. R. Armstrong and M. Slaski, Chem. Soc. Rev., 1993, 22, 305 RSC.
- T. Kodaira, Y. Nozue, S. Ohwashi, T. Goto and O. Terasaki, Phys. Rev. B, 1993, 48, 12245 CrossRef CAS.
- Y. Nozue, T. Kodaira, S. Ohwashi, T. Goto and O. Terasaki, Phys. Rev. B, 1993, 48, 12253 CrossRef CAS.
- V. I. Srdanov, K. Haug, H. Metiu and G. D. Stucky, J. Phys. Chem., 1992, 96, 9309.
- L. R. M. Martens, P. J. Grobet and P. A. Jacobs, Nature, 1985, 315, 568 CrossRef CAS.
- J. A. Rabo, C. L. Angell, P. H. Kasai and V. Schomaker, Discuss. Faraday Soc., 1966, 41, 328 RSC.
- P. A. Anderson, R. J. Singer and P. P. Edwards, J. Chem. Soc., Chem. Commun., 1991, 914 RSC.
- P. A. Anderson and P. P. Edwards, J. Chem. Soc., Chem. Commun., 1991, 915 RSC.
- P. A. Anderson, D. Barr and P. P. Edwards, Angew. Chem., Int. Ed. Engl., 1991, 30, 1501 CrossRef.
- B. Xu and L. Kevan, J. Chem. Soc., Faraday Trans., 1991, 87, 2843 RSC.
- G. Engelhardt, M. Feuerstein, P. Sieger, D. Markgraber, G. Stucky and V. Srdanov, Chem. Commun., 1996, 729 RSC.
- H. Nakayama, D. D. Klug, C. I. Ratcliffe and J. A. Ripmeester, J. Am. Chem. Soc., 1994, 116, 9777 CrossRef CAS.
- The parent zeolite Na-A has a pseudo unit cell composition of Na12Al12Si12O48..
- P. A. Anderson and P. P. Edwards, J. Am. Chem. Soc., 1992, 114, 10608 CrossRef CAS.
- A. R. Armstrong, P. A. Anderson and P. P. Edwards, J. Solid State Chem., 1994, 111, 178 CrossRef CAS.
- NMR experiments were performed on samples sealed inside quartz MAS rotor inserts (Fluorochem/Wilmad) with epoxy. 29Si MAS spectra were measured at 71.5 MHz, at spinning speeds of up to 4 kHz, using a Chemagnetics 6 mm MAS probe in a Bruker MSL-360 spectrometer. Chemical shifts were measured relative to TMS. Field-dependent 27Al MAS spectra were collected at 93.8 MHz as above, and at 156.4 MHz using a 9.5 mm Chemagnetics probe in a Varian/Chemagnetics Infinity 600 spectrometer. 27Al shifts were measured relative to aqueous AlCl3..
- Rietveld refinement of powder neutron diffraction data from these samples is in progress..
- L. J. Woodall, P. A. Anderson, A. R. Armstrong and P. P. Edwards, J. Chem. Soc., Dalton Trans., 1996, 719 RSC.
- R. Dupree, S. C. Kohn, C. M. B. Henderson and A. M. T. Bell, NATO ASI Ser., Ser. C, 1993, 386, 421 Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
