Photo-induced tautomerisation of methyltrioxorhenium(VII): the intermediate in olefin metathesis?
Leigh J. Morrisa, Anthony J. Downsa, Tim M. Greenea, G. Sean McGradyb, Wolfgang A. Herrmannc, Peter Sirschc, Odd Gropend and Wolfgang Schererd
aInorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX1 3QR. E-mail: tony.downs@chem.ox.ac.uk
bDepartment of Chemistry, King’s College London, Strand, London, UK WC2R 2LS
cAnorganisch-chemisches Institut, Technische Universität München, Lichtenbergstrasse 4, D-85747, Garching bei München, Germany
dInstitute of Mathematical and Physical Sciences, University of Tromsø, N-9037, Tromsø, Norway
First published on UnassignedUnassigned7th January 2000
Abstract
Matrix-isolated [CH3ReO3] tautomerises
to [H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH] under the influence of UV
light; the carbene has been characterised in its normal and 2H-
and 13C-enriched isotopic forms by its IR spectrum with results
well replicated by quantum chemical calculations.
Re(O)2OH] under the influence of UV
light; the carbene has been characterised in its normal and 2H-
and 13C-enriched isotopic forms by its IR spectrum with results
well replicated by quantum chemical calculations.
Since methyltrioxorhenium(VII), [CH3ReO3] 1, was first described, its rôle in promoting and catalysing numerous organic reactions has been explored in detail.1,2 In fact, 1 is probably the most widely active organometallic catalyst reported to date. This activity encompasses two general areas: (i) oxidation reactions (including olefin epoxidation, Baeyer–Villiger and aromatic oxidation);2–5 and (ii) olefin isomerisation and metathesis.6 The peroxo derivatives of 1 active in many reactions of type (i) have been isolated and characterised both structurally and spectroscopically.7 By contrast, the species responsible for catalytic activity of type (ii) have eluded direct detection, although a tautomer of the form [H2C
![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH] 2, has long
been presumed to be the active form of 1.2,8 There are numerous reports of rhenium–carbon
multiple bonds in the literature generally, but not exclusively, involving
rhenium in formal oxidation states <+7.8,9
Re(O)2OH] 2, has long
been presumed to be the active form of 1.2,8 There are numerous reports of rhenium–carbon
multiple bonds in the literature generally, but not exclusively, involving
rhenium in formal oxidation states <+7.8,9Photolysis of 1 in solution appears to result in homolysis of the Re–C bond.10 Our studies reveal, however, that the matrix-isolated molecule exhibits altogether different behaviour, initially tautomerising to the previously unknown carbene derivative 2 under the influence of UV light at wavelengths near 254 nm (Scheme 1). Tautomer 2 is also photolabile, broad-band UV–VIS irradiation (λ = 200–800 nm) causing it to decay to a product containing an Re–CO fragment, 3, possibly [H2Re(O)(OH)CO].
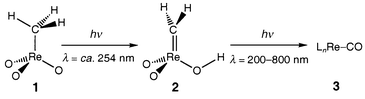 | ||
| Scheme 1 | ||
Exposure of 1 isolated in an Ar matrix at 14 K to UV radiation
with λ = ca. 254 nm for several minutes results in the
decay of the IR absorptions due to 1 and the simultaneous
appearance and growth of new absorptions apparently due to a single product
2 (Fig. 1). Irradiation of the
matrix with broad-band UV–VIS light (λ = 200–800 nm) was
observed to cause the evolution from 2 of at least one further
product 3 which could not be conclusively identified by its IR
spectrum. The IR bands identified on the evidence of their growth-decay
patterns enable 2 to be characterised as
[H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH], the conclusions being
underpinned (i) by the observed effects of 2H- and
13C-enrichment of the products derived from the species
[CD3ReO3] 1-d3,
and [13CH3ReO3] 1-13c, (ii) by parallels with the spectra of
related carbene and Re
Re(O)2OH], the conclusions being
underpinned (i) by the observed effects of 2H- and
13C-enrichment of the products derived from the species
[CD3ReO3] 1-d3,
and [13CH3ReO3] 1-13c, (ii) by parallels with the spectra of
related carbene and Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O derivatives, e.g.
CoCH211 and
ReO2F3,12 and (iii)
by comparisons with the results of Density Functional Theory (DFT)
calculations. (Calculations were carried out in Gaussian 9813 with geometries optimised at the BPW91/LANL2DZ
level of theory; standard 6-31G(d,p) basis sets were used for C, O and H,
whilst the Re basis set was augmented with an additional f-type
polarisation function.) Prominent among its IR absorptions were those at
3650.0, 992.2, 963.3 and 668.4 cm−1 which are identifiable
by their frequencies, intensities and responses to 2H- and
13C-enrichment with the modes ν(O–H),
νs(Re
O derivatives, e.g.
CoCH211 and
ReO2F3,12 and (iii)
by comparisons with the results of Density Functional Theory (DFT)
calculations. (Calculations were carried out in Gaussian 9813 with geometries optimised at the BPW91/LANL2DZ
level of theory; standard 6-31G(d,p) basis sets were used for C, O and H,
whilst the Re basis set was augmented with an additional f-type
polarisation function.) Prominent among its IR absorptions were those at
3650.0, 992.2, 963.3 and 668.4 cm−1 which are identifiable
by their frequencies, intensities and responses to 2H- and
13C-enrichment with the modes ν(O–H),
νs(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O), νas(Re
O), νas(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) and
ν(Re–OH), respectively. The presence of the
Re
O) and
ν(Re–OH), respectively. The presence of the
Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CH2 unit is signalled by bands at 3079.6, 2985.8,
1320.9, 778.8, 756.4 and 627.9 cm−1 which we associate
with the modes detailed in Table 1; each
assignment is attested by analogy with the corresponding mode of
CoCH211 and by the 2H
and 13C isotopic shifts, although the description of the motion
is sometimes less than exact. The spectrum is well simulated by a scaled
force field computed for 2 on the basis of DFT calculations
(Table 1), the 36 frequencies measured
for 2, 2-d3 and
2-13c being matched with an r.m.s. deviation
of only 1.66%. It is also evident that mixing of the ν(Re
CH2 unit is signalled by bands at 3079.6, 2985.8,
1320.9, 778.8, 756.4 and 627.9 cm−1 which we associate
with the modes detailed in Table 1; each
assignment is attested by analogy with the corresponding mode of
CoCH211 and by the 2H
and 13C isotopic shifts, although the description of the motion
is sometimes less than exact. The spectrum is well simulated by a scaled
force field computed for 2 on the basis of DFT calculations
(Table 1), the 36 frequencies measured
for 2, 2-d3 and
2-13c being matched with an r.m.s. deviation
of only 1.66%. It is also evident that mixing of the ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C),
ρ(CH2) and δ(OH) motions complicates the
interpretation of the spectrum in the region 600–800
cm−1, preventing the identification of any one feature
with the ν(Re
C),
ρ(CH2) and δ(OH) motions complicates the
interpretation of the spectrum in the region 600–800
cm−1, preventing the identification of any one feature
with the ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C) mode. The relative intensities of the bands due
to the well defined modes νas(Re
C) mode. The relative intensities of the bands due
to the well defined modes νas(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) and
νs(Re
O) and
νs(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O) imply an O
O) imply an O![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re
Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O angle in the
order of 116°, in good agreement with the optimum geometry computed for
2.
O angle in the
order of 116°, in good agreement with the optimum geometry computed for
2.
![(a) IR spectrum of [CH3ReO3] 1 isolated
in an argon matrix at 14 K; (b) IR spectrum showing the effect of
irradiation at λ = 254 nm for 15 min (↓ indicates a feature
associated with [H2CRe(O)2OH] 2);
and (c) the spectrum of 2 based on the results of DFT
calculations.](/image/article/2000/CC/a907908d/a907908d-f1.gif) | ||
Fig. 1 (a) IR spectrum of [CH3ReO3] 1 isolated
in an argon matrix at 14 K; (b) IR spectrum showing the effect of
irradiation at λ = 254 nm for 15 min (↓ indicates a feature
associated with [H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH] 2);
and (c) the spectrum of 2 based on the results of DFT
calculations. Re(O)2OH] 2);
and (c) the spectrum of 2 based on the results of DFT
calculations. | ||
| H212CReO2(OH) | H213CReO2(OH) | D212CReO2(OD) | ||||
|---|---|---|---|---|---|---|
| Obs. | Calc.b | Obs. | Calc.b | Obs. | Calc.b | Description of mode |
| a Frequencies in cm−1; all intensities normalised to that of the most intense band set equal to 100 (in parentheses).b Calculated frequency scaled by a factor of 0.9740. The r.m.s. deviation between observed and scaled calculated frequencies is 1.66%.c Indicates a feature too weak to be observed. | ||||||
| 3650.0 (100) | 3669.6 (87) | 3650.4 (100) | 3669.6 (87) | 2694.2 (73) | 2672.3 (63) | ν(O–H) |
| 2985.8 (3) | 2994.4 (5) | 2980.2 (4) | 2989.0 (5) | 2195.1 (5) | 2171.4 (6) | νs(CH2) |
| 1320.9 (2) | 1295.0 (5) | 1311.8 (2) | 1286.7 (5) | 1009.9 (2) | 1018.8 (6) | δ(CH2) |
| 992.2 (25) | 988.4 (30) | 991.9 (26) | 988.3 (30) | 992.2 (31) | 987.1 (42) | νs(ReO2) |
| 799.2 (2) | 815.4 (8) | 798.7 (3) | 812.4 (9) | 622.6 (3) | 631.0 (3) ![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) | |
| 778.8 (34) | 792.0 (10) | 768.3 (53) | 770.4 (14) | 578.9 (43) | 556.8 (81) | δ(O–H) + ρ(CH2) |
| 756.4 (30) | 741.9 (73) | 739.6 (19) | 736.5 (67) | 685.9 (10) | 698.2 (6) | + ν(Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) C) +
ν(Re–OH) C) +
ν(Re–OH) |
| 668.4 (83) | 664.2 (100) | 668.8 (87) | 664.2 (100) | 660.0 (69) | 664.7 (75) ![[lower bond 1 end]](https://www.rsc.org/images/entities/char_e013.gif) | |
| c | 286.4 (2) | c | 286.4 (2) | c | 286.0 (2) | ReO2 wag |
| c | 255.6 (1) | c | 255.6 (1) | c | 252.7 (2) | ReO2 scissor |
| c | 239.8 (3) | c | 239.8 (3) | c | 217.6 (3) | δ(CReOH) |
| 3079.6 (3) | 3091.9 (0.6) | 3068.4 (1) | 3079.5 (0.6) | 2315.2 (1) | 2299.0 (0.3) | νas(CH2) |
| 963.3 (64) | 967.2 (81) | 963.4 (80) | 967.1 (81) | 961.8 (100) | 965.0 (100) | νas(ReO2) |
| 627.9 (3) | 644.2 (1) | 625.7 (5) | 639.3 (2) | 500.2 (2) | 504.1 (0.3) | CH2 scissor |
| c | 526.4 (0.2) | c | 525.8 (0.2) | c | 386.1 (0.01) | CH2 wag |
| 321.2 (33) | 315.9 (42) | 320.7 (38) | 315.2 (43) | 239.3 (19) | 253.4 (6) | δ(O–H) |
| c | 266.8 (15) | c | 266.8 (15) | c | 237.3 (15) | δ(CReO2) |
| c | 233.2 (0.03) | c | 233.2 (0.03) | c | 208.5 (16) | d(CReO2) |
Similar experiments with [CH2DReO3]
1-d1 give rise not only to
[H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OD], but also to the isotopomer
[H(D)C
Re(O)2OD], but also to the isotopomer
[H(D)C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH]
2-d1. Here the ν(C–H)
fundamental is isolated from all other modes in the molecule and so gives
access, in principle, to relatively precise estimates of the dimensions of
the CH2 unit.14 The measured
value of νis(C–H) (3035.4 cm−1), taken
together with ν(12C–H), ν(13C–H)
and ν(12C–D) data for the other isotopomers, affords
values that tally with the results of the DFT optimisations given in
parentheses: r0 = 1.088 Å
(re = 1.099 Å), ∠HCH = 119 ±
4° (∠eHCH = 115.8°).
Re(O)2OH]
2-d1. Here the ν(C–H)
fundamental is isolated from all other modes in the molecule and so gives
access, in principle, to relatively precise estimates of the dimensions of
the CH2 unit.14 The measured
value of νis(C–H) (3035.4 cm−1), taken
together with ν(12C–H), ν(13C–H)
and ν(12C–D) data for the other isotopomers, affords
values that tally with the results of the DFT optimisations given in
parentheses: r0 = 1.088 Å
(re = 1.099 Å), ∠HCH = 119 ±
4° (∠eHCH = 115.8°).
Although 2 was formed almost exclusively when matrix-isolated
1 was photolysed at wavelengths near 254 nm, exposure to
broad-band UV–VIS light gave rise to a secondary change. The sole
detectable product 3 formed from 2 under these
conditions, but always in the presence of an abundance of 1 and
2, could be identified by a single IR band at 2051.4
cm−1. Assignment to the ν(C–O) mode of an
Re–CO moiety is strongly urged by a minimal change of frequency when
3 is formed from 1-d3 or
1-d1 but by a shift to 2003.8
cm−1 when it is formed from
1-13c. The circumstances preclude positive
identification, but a possible candidate for 3 is the novel
rhenium(V) compound [H2Re(CO)(O)OH] formed by
photoisomerisation of [H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH] in a
change that would parallel the conversion of [H2COSi] to
[H2Si∶CO].15 DFT
calculations provide some support for [H2Re(CO)(O)OH], finding a
potential energy minimum 126 kJ mol−1 above that of
[H2C
Re(O)2OH] in a
change that would parallel the conversion of [H2COSi] to
[H2Si∶CO].15 DFT
calculations provide some support for [H2Re(CO)(O)OH], finding a
potential energy minimum 126 kJ mol−1 above that of
[H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) Re(O)2OH] with a structure approximating
to a square-based pyramid having the unique oxide ligand at the apex, and a
calculated ν(C–O) frequency of 2040 cm−1
(12C/13C shift = 44.5 cm−1).
Re(O)2OH] with a structure approximating
to a square-based pyramid having the unique oxide ligand at the apex, and a
calculated ν(C–O) frequency of 2040 cm−1
(12C/13C shift = 44.5 cm−1).
[CH3ReO3] is active in olefin metathesis only when
activated by a co-catalyst (S4N4/AlCl3),
or when supported on silica or alumina.8 DFT
calculations indicate that 2 lies ca. 89 kJ
mol−1 higher in energy than 1, and hence is
inaccessible under normal thermal conditions. Model calculations on
[CH3ReO2{(η2-OSiH2)
2O}] 4, the product formed by condensation of
[CH3ReO3] with disilanol
([H2Si(OH)]2O), show the tautomeric H-atom transfer
to occur preferentially to an Re–O–Si bridging oxygen atom
rather than to an Re![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O unit. The resulting carbene species
[H2C
O unit. The resulting carbene species
[H2C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) ReO2{(η2-OSiH2
)(O)(SiH2OH)}] 5 is effectively stabilised
by ca. 48 kJ mol−1 relative to complex
4. This alternative H-atom transfer to the Re–O–Si
bridge would seem to be a more realistic mechanism for carbene formation on
supported [CH3ReO3].
ReO2{(η2-OSiH2
)(O)(SiH2OH)}] 5 is effectively stabilised
by ca. 48 kJ mol−1 relative to complex
4. This alternative H-atom transfer to the Re–O–Si
bridge would seem to be a more realistic mechanism for carbene formation on
supported [CH3ReO3].
References
- W. A. Herrmann, J. Organomet. Chem., 1995, 500, 149 CrossRef CAS.
- C. C. Romão, F. E. Kühn and W. A. Herrmann, Chem. Rev., 1997, 97, 3197 CrossRef CAS.
- W. A. Herrmann, R. W. Fischer and D. W. Marz, Angew. Chem., Int. Ed. Engl., 1991, 30, 1638 CrossRef.
- W. A. Herrmann, R. W. Fischer and J. D. G. Correira, J. Mol. Catal., 1994, 94, 213 CrossRef CAS.
- R. A. Sheldon, Top. Curr. Chem., 1993, 164, 21 Search PubMed.
- W. A. Herrmann, W. Wagner, U. N. Flessner, U. Volkhardt and H. Komber, Angew. Chem., Int. Ed. Engl., 1991, 30, 1636 CrossRef.
- A. M. Al-Ajlouni and J. H. Espenson, J. Org. Chem., 1996, 61, 3969 CrossRef CAS; M. M. Abu-Omar and J. H. Espenson, Organometallics, 1996, 15, 3543 CrossRef CAS.
- D. M. Hoffman, in Comprehensive Organometallic Chemistry II, ed. C. P. Casey, Pergamon, Oxford, 1995, vol. 6, p. 231. Search PubMed.
- J. M. O’Connor, in Comprehensive Organometallic Chemistry II, ed. C. P. Casey, Pergamon, Oxford, 1995, vol. 6, p. 200. Search PubMed.
- H. Kunkely, T. Türk, C. Teixeira, C. de Meric de Bellefon, W. A. Herrmann and A. Vogler, Organometallics, 1991, 10, 2090 CrossRef CAS.
- W. E. Billups, S.-C. Chang, R. H. Hauge and J. L. Margrave, J. Am. Chem. Soc., 1995, 117, 1387 CrossRef CAS.
- I. R. Beattie, R. A. Crocombe and J. S. Ogden, J. Chem. Soc., Dalton Trans., 1977, 1481 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Lui, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, Gaussian 98, Revision A.3, Gaussian Inc., Pittsburgh PA, 1998. Search PubMed.
- D. C. McKean, Chem. Soc. Rev., 1978, 7, 399 RSC; Croat. Chem. Acta, 1988, 61, 447. Search PubMed.
- G. Maier, H. P. Reisenauer and H. Egenolf, Organometallics, 1999, 18, 2155 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2000 |
