Trace element determination of single fluid inclusions by laser ablation ICP-MS: applications for halites from sedimentary basins
A. Mohamad Ghazi*a and Stephen Shuttleworthb
aDepartment of Geology, Georgia State University, Atlanta, GA 30303, USA.. E-mail: mghazi@gsu.edu
bMerchantek EO, P.O. Box 2277, Del Mar, CA 92014, USA.. E-mail: 51510@aol.com
First published on UnassignedUnassigned7th January 2000
Abstract
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) was used to determine Ca, Sr and Rb in individual natural fluid inclusions in halite. Artificial fluid inclusions made in glass microcapillary tubes were used to quantify the data. An Nd:YAG laser operating at 266 nm was focused and fired in Q-switched mode on 20–30 μm diameter spots on the microcapillary tubes until the fluid was reached. The standard fluid was ablated and transported with argon to the ICP-MS. Analytes in fluid inclusions in the halite were determined using calibration curves obtained from standard solutions in the microcapillaries. Uncertainties in the analysis of individual fluid inclusions ranged from 4% to 20% RSD for samples from the Palo Duro and Paradox Basins in Texas and Utah, respectively. Ca and Sr in halite from the Palo Duro Basin ranged from 95 to 1028 ppm and from 6.7 to 607 ppm, respectively. Halite from the Paradox Basin contained fluid inclusions with 124 to 178 ppm Ca and 14 to 108 ppm Sr. In all inclusions, Ca had the highest concentration, followed by Sr and Rb. Ca and Sr concentrations for fluid inclusions in halite from the Palo Duro Basin were generally greater than those from the Paradox Basin, and were greater with depth in the basin.
Introduction
The compositions of fluid inclusions in minerals provide a valuable source of information for inspecting the conditions of formation of fluid–rock systems. Fluid inclusions can be analysed by a variety of non-destructive and destructive techniques.1 Non-destructive methods that have been successfully used for the analysis of major elements in single inclusions include: synchrotron X-ray fluorescence, proton-induced X-ray emission and proton-induced gamma ray emission. Destructive techniques typically provide greater compositional information on the fluid inclusions and are more widely available than the non-destructive techniques. Analysis of single fluid inclusions by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) is considered to be a destructive method.2–6 ICP-MS has also been used for elemental analysis in bulk fluid extracted by crush–leach or decrepitation of fluid from inclusions.7,8The objectives of the present paper were to evaluate the experimental design and validity of using artificial fluid inclusions in microcapillary tubes as an external standard method for LA-ICP-MS, and to present detailed information on the elemental composition (e.g., Ca, Sr and Rb) from individual fluid inclusions in halite samples from the Palo Duro Basin, Texas and Paradox Basin, Utah. This type of analysis is necessary to answer many important questions about the past history of sedimentary basins. For example, it may indicate changing provenances for certain groups of elements (e.g., Ca, Sr, Ba and Rb) during deposition of the basin, or may record changing processes for halite precipitation over time in a single basin. Additional studies were also carried out using a Merchantek EO-LUV (Del Mar, CA, USA) 266 Nd:YAG laser.
Experimental
LA-ICP-MS system
The laser ablation system used in this study is the Finnigan-MAT (San Jose, CA, USA) System 266 Nd:YA laser. The laser operates with a fundamental IR wavelength of 1064 nm which is quadrupled to UV using two sets of crystals to 266 nm. The 266 nm range is preferred because of its better coupling with IR transparent materials such as halite, quartz and glass. The laser operates in Q-switched mode at pulse rates of 1–12 Hz, delivering a maximum energy of about 5 mJ pulse−1 at 266 nm. The samples and standards are attached to a glass slide with two-sided tape and placed in a ≡150 ml cylindrical sample stage composed of Pyrex sides with openings for the introduction and escape of the argon carrier gas, and a quartz glass top at an angle of ≡30° through which the laser beam travels to reach the sample.For this study, a Finnigan-MAT SOLA ICP-MS, located at Georgia State University, was used. In contrast to most laser ICP-MS configurations, a dual gas flow system is used which allows mixing of the ablated material with ultrapure water which is introduced into the torch through a peristaltic pump just prior to ionization. This configuration reduces background and improves signal stability for the ICP-MS. The quadrupole mass spectrometer contains two detectors, a Faraday cup for large signals, and a secondary electron multiplier (SEM) detector which is positioned off-axis, negating the necessity of a photon stop in the ion path. Typical operating conditions for data acquisition are summarized in Table 1.
| System 266 laser— | |
| Laser operating mode | Q-switched |
| Repetition rate | 4 Hz |
| Output power | 4 mJ pulse−1 |
| Ablation pit size | 20 μm diameter |
| Focus condition | Focus on sample surface |
| Gas flow rate | 0.3 l min−1 |
| SOLA ICP-MS— | |
| Nebulizer flow rate | 0.627 l min−1 |
| Acquisition mode | Scanning |
| Faraday scanning conditions | 1 scan, 4 passes |
| SEM scanning conditions | 1 scan, 4 passes |
| Dwell time (Faraday) | 16 ms |
| (SEM) | 64 ms |
| Acquisition time (Faraday) | 8 s |
| (SEM) | 23 s |
| Resolution (mass) | 16 channels amu−1 |
Description of samples
Fluid inclusions in halite are from the Palo Duro Basin, which is one of five Permian structural basins in Texas,9 and from the Paradox Basin, located within the southwest corner of Colorado and southeastern corner of Utah. The geological setting of the Palo Duro and Paradox Basins and their depositional history have been studied in detail.9–11 The fluid inclusion-bearing chips are from core samples. Samples from the Palo Duro Basin are from ≡786 m and ≡819 m below the surface, and the Paradox Basin halite is from ≡166 m below the surface. In general, the samples contain two types of fluid inclusions: small inclusions (30–70 μm) which are typically cubic and form chevron-like structures that are present near the intergranular crystal boundaries, and larger inclusions which are typically isolated and are up to 1 mm in diameter. In this study, we analysed the larger inclusions only.Artificial fluid inclusion standards
A set of artificial standards was prepared by drawing 0.2–0.3 ml of a standard solution of known composition (1000 ppm, 500 ppm, 250 ppm or 125 ppm of Ca, Sr and Rb in 2% HNO3) into 4 μl−1 volume glass microcapillary tubes (Microcaps, Drummond Scientific, Broomall, PA, USA). The multi-element standard solutions were prepared by mixing of single-element SPEX standard solutions (SPEX Industries, Metuchen, NJ, USA). After the solution is drawn into the tube by capillary action, the liquid column is moved to the center of the tube by suction at one end, leaving a void volume at both ends of the tube (Fig. 1), and the ends are then carefully sealed at both ends with a jeweler’s torch using a very fine flame. | ||
| Fig. 1 Photomicrograph of a 4 μl volume microcapillary tube. Distance AB shows the length of an artificial fluid inclusion that contains the Ca, Sr and Rb multi-element standard solution. | ||
At distances of about 400–500 μm from one end (meniscus) of the liquid column, the laser is fired in Q-switched mode to ablate through the wall of the microcapillary tube. During ablation, isotopes of 44Ca, 88Sr and 83Rb are continually monitored by recording the signal intensity at m/z 44, 85 and 88 after each scan. The initial contact of the laser with the standard solution is marked by a jump in signal intensities above background levels. A number of measurements are then taken, until the fluid between the meniscus and the firing hole is virtually ablated to the point that the edge of the liquid meniscus reaches the hole created by the laser, which is indicated by a sharp drop of signal intensities back to background level. A new ablation spot is then selected at a similar distance from the previous hole and a new set of measurements is obtained, following the same procedure. During the ablation process, one can clearly monitor the behavior of the liquid and the movement of the liquid meniscus toward the firing hole. The wall thickness and inner diameter of the microcapillary tubes are known to within 1%, permitting an estimate of the average volume of ablated fluid per pulse of 90–100 pl pulse−1 at 4 Hz.4
Results and discussion
Fluid inclusion standards
One concern in laser ablation of glass microcapillary tubes is the possibility of fracturing or exploding of the tubes. However, the resulting holes are smooth (Fig. 2A), and it appears that the laser ablates through the glass without breaking or damaging the internal portions of the microcapillary tube, although mechanical damage can occur at the surface (Figs. 2B, C). It is also evident that the diameter of the beam decreases by roughly 40% over a depth of 200 μm. However, once the beam reaches the liquid, the diameter remains constant and equal to the minimum diameter of the hole at the point of contact with the fluid (Fig. 2A). In contrast to solids, consistent volumes of liquid are ablated volume of solution between each pulse. Thus, for inclusions with sufficiently large volumes, numerous measurements can be taken without decay of signal with depth as can occur in solids analysis.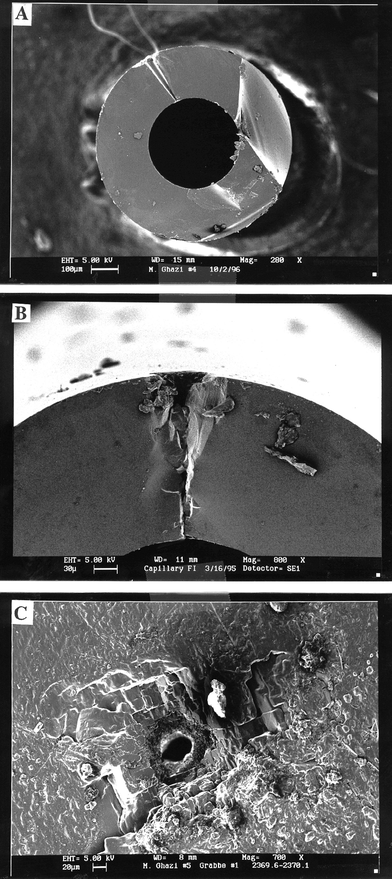 | ||
| Fig. 2 (A) SEM photomicrograph of a 4 μl volume microcapillary tube showing in cross-section one of the laser holes which has completely cut through the thickness of the wall of the microcapillary tube. (B) SEM photomicrograph showing mechanical damage (conchoidal fracture) in a cross-section of a microcapillary tube which indicates that the removal of solution is almost exclusively by mechanical forces rather than by vaporization. (C) SEM photomicrograph showing mechanical damage (three perfect cleavages) in a halite sample. | ||
Two different methods are used to report the precision for the standards. The short-term precision for a specific standard is the relative standard deviation (RSD in %) obtained from analyses of the solution ablated through an individual hole, and the long-term precision is the RSD% for analyses of intensity signals from all holes in a single microcapillary tube. For example, the long-term RSD% for Rb and Sr signals varies between 1.41% for the 125 ppm Rb solution and 2.24% for the 1000 ppm Rb solution and from 2.9% for the 125 ppm Sr solution to 2.69% for the 1000 ppm solution. The precision for Ca analyses varies between 7.18% for the 125 ppm solution to 1.77% for the 250 ppm solution (Table 2). In general, the short-term precision varies between 4 and 16%. Concentrations of Ca and Sr in the natural fluid inclusions were determined using linear calibration curves calculated from the standards (Fig. 3). In this method, a calibration curve was created by plotting the signal intensity (counts per second) from the analysis of 1000, 500, 250 and 125 ppm for Ca and 1000, 250 and 125 ppm for Sr and Rb artificial fluid inclusions versus concentration (ppm). The final calculation for concentration was made using the average values for the peak intensity data for each natural inclusion (Table 3) and a linear equation that best fits the appropriate calibration curve.
| Rb | Sr | Ca | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 125 ppm | 250 ppm | 1000 ppm | 125 ppm | 250 ppm | 1000 ppm | 125 ppm | 250 ppm | 500 ppm | 1000 ppm | |
| Intensity | 101262 (16) | 196595 (11) | 606166 (22) | 97873 (16) | 206106 (11) | 748108 (22) | 20075 (29) | 36809 (27) | 91406 (8) | 221675 (11) |
| 100708 (16) | 191989 (11) | 587261 (22) | 99127 (16) | 212324 (11) | 759022 (22) | 19542 (41) | 35898 (31) | 95122 (28) | 193834 (11) | |
| 100242 (16) | 200089 (11) | 95473 (16) | 178291 (11) | 721164 (22) | 19759 (41) | 94963 (20) | 202692 (29) | |||
| 98566 (16) | 185227 (11) | 96456 (16) | 193064 (11) | 719022 (22) | 22705 (41) | 88106 (27) | 206403 (29) | |||
| 99375 (16) | 98968 (16) | 87712 (20) | ||||||||
| 101450 (16) | 103953 (16) | 98012 (20) | ||||||||
| 102288 (16) | 101194 (16) | |||||||||
| Mean | 100642 | 193475 | 596714 | 99006 | 197447 | 736829 | 20520 | 36354 | 92399 | 206151 |
| SD | 1423.7 | 6421.9 | 13367.9 | 2873.7 | 15082.4 | 19851.8 | 1472.8 | 643.8 | 3337.1 | 11615.1 |
| RSD% | 1.41 | 3.32 | 2.24 | 2.90 | 7.64 | 2.7 | 7.18 | 1.8 | 3.6 | 5.6 |
 | ||
| Fig. 3 Calibration curves (concentration of known standard versus counts per second) used to calculate concentration of Ca, Sr and Rb in fluid inclusions in halite. | ||
| Inclusion no. | Ca/cps | RSD% | Sr/cps | RSD% | Rb/cps | RSD% | Ca/ppm | Sr/ppm | Ca/Sr |
|---|---|---|---|---|---|---|---|---|---|
| PD-GRA-1 | 30924 | 3.5 | 58622 | 6.4 | 564 | 1.9 | 188 | 64.4 | 2.3 |
| PD-GRA-2 | 85053 | 10.3 | 177003 | 6.1 | 177 | 8.2 | 442 | 227 | 1.9 |
| PD-GRA-3 | 17649 | 9.5 | 22031 | 7.4 | 1601 | 1.55 | 126 | 13.9 | 9.1 |
| PD-GRA-4 | 29290 | 14.8 | 45896 | 10.5 | 2228 | 13.8 | 180 | 46.9 | 3.9 |
| PD-GRA-5 | 11093 | 9.9 | 16750 | 12.3 | 1135 | 11.5 | 95.4 | 6.7 | 14.2 |
| PD-GRA-6 | 22085 | 18.7 | 22767 | 16.7 | 1592 | 15.2 | 146 | 14.9 | 9.9 |
| PD-JF-1 | 185588 | 14.9 | 281900 | 12.8 | 344 | 25.8 | 913 | 380 | 2.4 |
| PD-JF-2 | 44078 | 3.5 | 84710 | 10.6 | 3180 | 16.4 | 250 | 100 | 2.5 |
| PD-JF-3 | 104641 | 9.9 | 193172 | 17.2 | 8366 | 9.4 | 534 | 249 | 2.1 |
| PD-JF-4 | 168348 | 5.8 | 227722 | 4.3 | 2886 | 13.8 | 832 | 297 | 2.8 |
| PD-JF-5 | 38373 | 10.8 | 54280 | 42.2 | 2088 | 39.8 | 223 | 58.1 | 3.8 |
| PD-JF-6 | 210016 | 13.6 | 452551 | 11.2 | 1682 | 12.9 | 1028 | 607 | 1.7 |
| PA-GDI-1 | 20924 | 19.8 | 24799 | 18.2 | 2590 | 15.2 | 143 | 17.8 | 8.1 |
| PA-GDI-2 | 17197 | 16.8 | 22527 | 9.4 | 2695 | 10.2 | 124 | 14.6 | 8.5 |
| PA-GDI-3 | 20965 | 19.3 | 90539 | 5.8 | 4431 | 1.8 | 141 | 108 | 1.3 |
| PA-GDI-4 | 28803 | 16.5 | 34348 | 5.8 | 3437 | 8.5 | 178 | 30.9 | 5.8 |
Natural fluid inclusions in halite
Halite samples were selected for analysis because they contain fluid inclusions with large volumes that do not include solid phases. Thus, it is possible to analyse the natural inclusions under analytical conditions very similar to those used for microcapillary fluid inclusions. Also, Sr concentration data obtained by other means are available for fluid inclusions from the Palo Duro Basin,9 permitting a comparison between our results and those obtained by other methods by earlier workers. In all analyses of halite fluid inclusions in halite, the transition in signal intensity from the ablation of host halite to liquid inclusions is marked by a significant increase (up to six orders of magnitude) in peak intensities (Fig. 4). These observations demonstrate that virtually all the Ca and Sr reside in the fluid inclusion and are being efficiently extracted in the ablation process. Spatial variations are also noted in abundances and ratios between Ca and Sr among individual inclusions. Figs. 4A and 4B show the entire sequence of events during the analysis of a single fluid inclusion in two different samples. Approximately 40 s were required for the laser beam to penetrate through the halite to reach the fluid inclusions. The opening of the inclusion is marked by a significant increase in signal intensity for all three isotopes (e.g., in one case the increase in signal intensity is as large as six orders of magnitude). Ablation of the fluid is shown as a broad peak between approximately 40 and 80–90 s (Figs. 4A and 4B). Once the fluid is completely ablated, the signal intensities for all three elements drop back to the level for the host halite. In the case of the two-phase inclusions (L + V), the response of the liquid (L) to each pulse of laser is evident in the form of rapid movement of the vapor (V) bubble within the inclusion. Furthermore, the vapor bubble continues to grow larger at the expense of the ablated liquid solution from the inclusion. | ||
| Fig. 4 Time-resolved diagrams for six natural fluid inclusions in halite from Palo Duro Basin, Texas and Paradox Basin, Utah. | ||
Figs. 4A and 4B represent the ideal situation for single fluid inclusion analysis, whereas more complex spectra require more careful interpretation. For example, there are cases where two sequences of fluid release appear to have taken place. These sequences result from the interaction of two inclusions at two different depths along the beam path (Fig. 4C), or if the fluid inclusion is deeply situated, as in Fig. 4D where nearly 120 s are required for the beam to reach the fluid inclusion. Fig. 4E represents the spectrum for a large, but shallow inclusion (only 15 s to reach the inclusion), that after 100 s of ablation was not completely emptied. Fig. 4F represents the spectrum for a very shallow inclusion, virtually opened at the time of initial ablation in which Ca and Sr are equally abundant.
The analyses of fluid inclusions also indicate a spatial variation in elemental concentrations within a population of fluid inclusions, as shown by the variability in signal intensity for the different inclusions. It is important to note that these variations do not correlate with depth from the surface of the halite sample to the inclusion, which is expressed as the time required for the fluid inclusion to be reached. For example, sample PD-GRA-2 has an Sr signal intensity nearly twice the intensity of sample PD-JF-2, even though it took over 100 s to reach the fluid, compared to 40 s for PD-JF-2 (compare Figs. 4A and 4D). In contrast, samples PD-GRA-1 and PD-GRA-6 have nearly identical Ca intensities, with the latter inclusion ablated at t = 0 s, whereas PD-GRA-1 required 70 s to be reached (Figs. 4C and 4F). These results suggest that variations in intensity cannot be solely an artifact of depth from the surface. The data clearly distinguish between signals that are obtained from the host halite and those from fluid ablated from inclusions. It is also clear that changes in the magnitude of the signal intensity are simultaneous for all three isotopes, as the laser beam ablates through the mineral to the fluid inclusions and then back to the host mineral. This phenomenon further demonstrates that higher peak intensities are obtained from analysis of liquid that contains a higher concentration of a particular element.
Means, standard deviations (SD) and relative standard deviations (RSD) of Ca and Sr concentrations for 16 individual fluid inclusions are summarized in Table 3. The reproducibility of the results from halite-hosted fluid inclusions is less precise than those from artificial fluid inclusions. The RSD for Ca analyses ranges from 3.5 to 20%, whereas Sr ranges from 4.3 to 18.2% (in one case 42.2%) and Rb ranges from 1.55% to 25.8%. The larger RSD may be statistically induced, as fewer scans (3–5) are obtained owing to the smaller volumes of fluid available for ablation relative to the standards. They may also be due to the physical differences between liquid fluid in the inclusions and the solid host mineral. Halite is softer than glass and has three perfect cleavages, which could result in an irregular geometry of the ablation hole. An irregular geometry may in some ways inhibit escape of the ablated fluid inclusions, although observations suggest that a cylindrical hole is formed in halite shortly after ablation (Fig. 2C).
Calcium concentrations range from 95.4 to 1028 ppm for samples from the Palo Duro Basin and from 124 to 178 ppm for samples from the Paradox Basin. Similarly, concentrations for Sr range from 6.7 to 607 ppm for the Palo Duro inclusions and from 14.6 to 108 ppm for the Paradox inclusions. In general, these results suggest that the average concentrations of Ca and Sr in flud inclusions from the Palo Duro Basin are greater than those from the Paradox Basin. The results also strongly suggest that, in the Palo Duro Basin, there are spatial variations of Ca and Sr in populations of fluid inclusions from different depths. For example, all of the inclusions from greater depths in the Palo Duro Basin have higher concentrations of both Ca and Sr than those from shallower depth.
At the Palo Duro Basin, Sr concentrations reported in this study (58–607 ppm for PD-JF and 6.7–227 ppm for PD-GRA) are similar to those determined by decrepitation methods, which range from 33 to 230 ppm.9,10Figs. 5A and 5B represent the variations of Sr and Ca with depth in two of the core samples from the Palo Duro Basin. The figures show that inclusions from the shallower depth (PD-GRA) in general have lower concentrations and less variation. Also, Ca/Sr values are more restricted at depth for the Palo Duro samples, and the deeper samples are similar to those from the Paradox Basin (Fig. 5C).
 | ||
| Fig. 5 (A and B) Sr and Ca concentration data frm Palo Duro Basin versus depth. (C) Ca/Sr ratio data versus depth for Palo Duro and Paradox Basins. | ||
Conclusions
This work has shown that, using a combination of artificial fluid inlcusion standards in microcapillaries, laser ablation ICP-MS can successfully be applied to quantitatively measure the concentrations of trace elements in natural fluid inclusions. Results of such analyses are essential to answer many significant questions about the past history of sedimentary basins. For example, they may indicate changing provenances for Ca and Sr during deposition of the basins, or may record changing processes for halite precipitation over time in a single basin. Moreover, such analyses also provide important information on the origin of the fluid and its evolution in the context of sedimentary and diagenetic environments in which it was formed, as well as information on the composition of seawater at the time of formation of the basins. Further analyses of larger populations of inclusions with a greater selection of elements (e.g., Mg, Ba, K) are warranted to establish an accurate geological interpretation and to help to identify primary inclusions for K/Ar isotopic analysis. Although the large range of variabilities in RSD associated with one analysis of a natural fluid inclusion requires that a number of inclusions be measured to obtain representative concentrations, the microcapillary standard approach for fluid inclusion analysis is a promising technique.Acknowledgements
We wish to thank Dr. Marion Wampler of Georgia Institute of Technology who kindly shared his halite samples from the Palo Duro and Paradox Basins. This research was supported by collaborative NSF grants EAR-95-06023 to Ghazi and Vanko and EAR-98-14754 to Ghazi. The analyses were performed at the GSU’s regional laser ablation ICP-MS laboratory, funded by NSF grant (EAR-94-05716) to A. M. Ghazi and D. A. Vanko.References
- E. Roedder, Reviews in Mineralogy, Mineralogical Society of America, Washington, DC, 1984, vol. 12, p. 644. Search PubMed.
- A. M. Ghazi, T. E. McCandless, J. Ruiz and D. A. Vanko, EOS., Trans. AGU, 1995, 76, 5287.
- A. Moissette, T. J. Shepherd and S. Chenery, J. Anal. At. Spectrom., 1996, 11, 177 RSC.
- A. M. Ghazi, T. E. McCandless, D. A. Vanko and J. Ruiz, J. Anal. At. Spectrom., 1996, 11, 667 RSC.
- A. Audetat, D. Gunther and C. A. Heinrich, Science, 1998, 279, 2091 CrossRef CAS.
- T. E. McCandless, D. J. Lajack, J. Ruiz and A. M. Ghazi, J. Geostand. Geoanal., 1998, 21, 279 Search PubMed.
- A. M. Ghazi, D. A. Vanko, E. Roedder and R. C. Seeley, Geochim. Cosmochim. Acta, 1993, 57, 4513 CrossRef CAS.
- D. A. Banks, B. W. A. Yardley, A. R. Campbell and K. E. Jarvis, Chem. Geol., 1994, 112, 259 CrossRef CAS.
- A. Bein, S. D. Hovorka, R. S. Fisher and E. Roedder, J. Sediment. Petrol., 1991, 61, 1 Search PubMed.
- E. Roedder, W. M. D’Angelo, A. F. Dorzapf Jr and P. J. Aruscavage, Chem. Geol., 1987, 61, 79 CrossRef CAS.
- D. R. Shaw, US Geological Survey Professional Paper 576-E, Geological Society of America, 1978. Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
