An electrochemiluminescence-based fibre optic biosensor for choline flow injection analysis
Valérie C. Tsafack†, Christophe A. Marquette, Béatrice Leca and Loïc J. Blum*
Laboratoire de Génie Enzymatique, UMR CNRS 5013, Université Claude Bernard Lyon 1, Bât. 308-43, bd du 11 Novembre 1918, F-69622, Villeurbanne Cedex, France.. E-mail: Loic.Blum@univ-lyon1.fr;; Fax: +33 472 44 28 34;; Tel: +33 472 43 13 97
First published on UnassignedUnassigned7th January 2000
Abstract
A fibre optic biosensor based on luminol electrochemiluminescence (ECL) integrated in a flow injection analysis (FIA) system was developed for the detection of choline. The electrochemiluminescence of luminol was generated by a glassy carbon electrode polarised at +425 mV vs. a platinum pseudo-reference electrode. Choline oxidase (Chx) was immobilised either covalently on polyamide (ABC type) or on UltraBind preactivated membranes, or by physical entrapment in a photo-cross-linkable poly(vinyl alcohol) polymer (PVA-SbQ) alone or after absorption on a weak anion exchanger, DEAE (diethylaminoethyl) Sepharose. The optimisation of the reaction conditions and physicochemical parameters influencing the FIA biosensor response demonstrated that the choline biosensor exhibited the best performances in a 30 mM veronal buffer containing 30 mM KCl and 1.5 mM MgCl2, at pH 9. The use of a 0.5 ml min−1 flow rate enabled the measurement of choline by the membrane-based ECL biosensors in 8 or 5 min, with ABC or UltraBind membranes, respectively, whereas the measurement required only 3 min with the DEAE–PVA system. For comparison, the detection of choline was performed with Chx immobilised using the four different supports. The best performances were obtained with the DEAE–PVA–Chx sensing layer, which allowed a detection limit of 10 pmol, whereas with the ABC, the UltraBind and the PVA systems, the detection limits were 300 pmol, 75 pmol and 220 pmol, respectively. The DEAE-based system also exhibited a good operational stability since 160 repeated measurements of 3 nmol of choline could be performed with an RSD of 4.5% whereas the stability under the best conditions was 45 assays with the other supports.
Introduction
Choline, found in tissues of a variety of organs such as liver, lung, kidney and placenta, is a precursor of the important neurotransmitter acetylcholine used in the transmission of brain impulses between nerves, muscles and organs. It plays a vital role in maintaining the central nervous system and numerous metabolic functions. A deficiency of acetylcholine could result in increasing fatty deposits in the liver, memory loss and poor muscle co-ordination. The development of rapid detection methods of choline and acetylcholine are thus a great challenge.Moreover, the inhibition of acetylcholinesterase by certain compounds, such as organophosphorus pesticides, led a number of authors to investigate the use of a sensitive choline detection method in conjunction with measurements of acetylcholinesterase inhibition, for environmental monitoring of toxic compounds released in the drinking water.1–3
Consequently, several biosensors for the detection of choline, involving amperometry,4–7 or chemiluminescence,8–10 have been described in the literature. Nevertheless, the recurrent biosensor problems of sensitivity, stability and interference, are always present.
All of these previously described analytical systems were based on the detection of hydrogen peroxide or redox mediator compounds, produced during the course of the choline oxidase (Chx) catalysed reaction (reaction 1).
 | (1) |
The reliability of the electrochemical detection in complex samples could be affected by the presence of electroactive compounds in the sample matrix. In order to lower the applied potential and thus, to minimise the electrochemical interferences from the matrix, direct electron transfer between the enzyme and a redox mediator could be used.11
Another way to bypass these interference problems is the detection of oxidase-generated hydrogen peroxide by the chemiluminescence of luminol catalysed by immobilised horseradish peroxidase.10,12,13
A third and unusual way to obtain highly sensitive hydrogen peroxide detection, is the electrogenerated chemiluminescence of luminol (ECL). In a mechanistic study of this electroluminescence reaction, Sakura proposed that luminol was first oxidised at the electrode surface and then reacted, mole to mole, with hydrogen peroxide.14 The theoretical ratio (photon produced)/(H2O2 consumed) was then 1, while it was 0.5 for the peroxidase-catalysed reaction. Consequently, regarding the sensitivity of hydrogen peroxide detection, the electrogenerated chemiluminescence of luminol might be more efficient than the peroxidase-catalysed luminescence.
The electrochemiluminescence of luminol in the presence of hydrogen peroxide has been recently explored for designing biosensors based on oxidase enzymes immobilised on artificial membranes.15 Such biosensors were shown to be sensitive, stable and free of complex matrix interference.
We thus investigate in this paper the use of this transduction system for a choline biosensor. The study points out the most effective immobilisation support for the achievement of a stable, rapid and sensitive detection of choline in flow injection analysis. Classical supports, such as preactivated membranes and photopolymer matrix were tested as well as a new immobilisation procedure involving the concomitant use of anion exchanger Sepharose beads and a poly(vinyl alcohol) bearing styrylpyridinium groups (PVA-SbQ) photopolymer.
Experimental
Reagents
Choline oxidase (Chx, EC 1.1.3.17, from Alcaligenes species, 17 U mg−1), choline chloride, luminol (3-aminophthalhydrazide) and bovine serum albumin (BSA, fraction V) were obtained from Sigma (St Quentin-Fallavier, France). Poly(vinyl alcohol) bearing styrylpyridinium groups (PVA-SbQ: polymerisation degree 2300, saponification degree 88, SbQ content 1.06%, solid content 11.10%, pH 6.2) was purchased from Toyo Gosei Kogyo (Chiba, Japan) and used as received. All buffer and aqueous solutions were prepared with distilled demineralised water. Luminol stock solution was made as 5.5 mM solution in 10−2 M KOH.Instrumentation and sensor assembly
The electrochemiluminescence measurements were performed in a flow system and recorded on a graphic recorder (Servotrace, Sefram, Saint-Etienne, France). The flow system consisted of a one channel peristaltic pump (model P-1, Pharmacia, Uppsala, Sweden), an injection valve (model 5020, Rheodyne, Cotati, CA, USA) on which a 30 μl sample loop was fitted and a specially designed flow cell with a 250 μl inner volume containing two magnetic bars (2 × 7 mm each) (Fig. 1). A liquid core single optical fibre from L.O.T. Oriel, Cortaboeuf, France, (core diameter 5 mm, overall diameter 7 mm) was connected to the photomultiplier tube of a luminometer (Biocounter M 2500, Lumac, Landgraaf, The Netherlands). The light intensity was expressed in arbitrary units (a.u.). The glassy carbon electrode (BAS, West Lafayette, IN, USA) was 3 mm in diameter and polarised at +425 mV vs. a pseudo-reference platinum electrode by a PRGE polarograph (Tacussel-Radiometer, Villeurbanne, France). In the biosensor configuration, the choline oxidase was immobilised either on preactivated membranes (polyamide-based ABC membrane, polyethersulfone-based UltraBind membrane) or in PVA-SbQ polymer, placed in close contact with the glassy carbon electrode. The carrier solution was thermostated at 30 °C and consisted of a suitable buffer, containing 50 μM of luminol.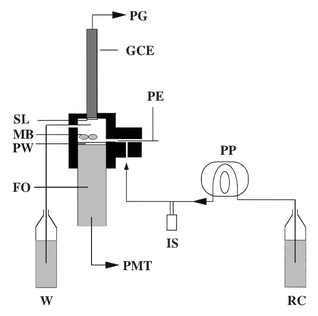 | ||
| Fig. 1 Schematic representation of the fibre optic continuous-flow system for electrochemiluminescence measurements. GCE, glassy carbon electrode; FO, fibre optic; IS, injection system; MB, magnetic bars; PE, platinum electrode; PG, polarograph; PMT, photomultiplier tube; PP, peristaltic pump; PW, Plexiglass window; RC, reagent carrier; SL, sensing layer; W, waste. Note that the different elements schematised are not strictly represented to the same scale. | ||
Enzyme immobilisation on preactivated membranes
UltraBind™ membranes (120 μm thickness and 0.45 μm pore diameter) and Immunodyne ABC membranes (120 μm thickness and 0.45 μm pore diameter) were supplied by Pall Gelman Sciences (Champs-sur-Marne, France) in a preactivated form directly usable for protein coupling. The membranes were cut out as 7 mm diameter disks and a specific immobilisation procedure was used for each type of membrane.The immobilisation on ABC type membranes was performed by applying 10 μl of a 10 mg ml−1 Chx solution in 0.1 M phosphate buffer pH 6, on each side of the disk. The coupling procedure was performed at room temperature and was complete after 5 min. The disks were then washed first for 20 min in 0.1 M phosphate buffer, pH 6, followed by 20 min in 0.1 M phosphate buffer, pH 8, containing 1 M KCl and 1% BSA, and finally 15 min in 0.1 M phosphate buffer, pH 6, with added 0.1 M KCl.
For the UltraBind membrane type, the immobilisation was performed according to a previously described procedure.16 Briefly, the membrane disks were dipped for 10 s in a 10 mg ml−1 Chx solution in 0.1 M phosphate buffer, pH 6, and dried for 15 min at room temperature. At that time, the immobilisation was complete and the Chx immobilised UltraBind membranes were washed in the same conditions as the ABC type membranes. Choline oxidase membranes were stored at −20 °C in 0.1 M phosphate buffer, pH 8, containing 0.1 M KCl, 1% BSA and 10% glycerol.
Choline oxidase entrapment in PVA-SbQ polymer
A solution of PVA-SbQ in water (1 + 9 ) containing 33 mg ml−1 of choline oxidase was prepared. A 25 μl portion of this solution was spread on the glassy carbon electrode and dried for 90 min under a tungsten lamp. The cast enzymatic layer was then exposed to ultraviolet light at 254 nm for 30 min in order to form the polymeric film in which choline oxidase was entrapped. The glassy carbon electrode supporting the film of choline oxidase was then washed for 20 min with 30 mM veronal buffer, pH 9, containing 30 mM KCl and 1.5 mM MgCl2 and stored dried at +4 °C.Choline oxidase immobilisation in DEAE-PVA
Prior to the binding of choline oxidase, 250 μl of DEAE Sepharose Fast Flow (average particle size 45–165), purchased from Pharmacia, were packed from ethanol slurry into a polyacrylamide column of appropriate volume. The DEAE gel was then equilibrated with 10 ml of 30 mM diethyl barbiturate (veronal) buffer containing 30 mM KCl and 1.5 mM MgCl2, pH 8.5. Thereafter, the gel was perfused with 1 ml of a 7 mg ml−1 enzyme solution in the equilibrating buffer which enabled the saturation of the DEAE gel. The unbound enzyme was then removed by washing the gel with 5 ml of equilibrating buffer. In the column thus obtained, the gel was saturated with choline oxidase and was stored at +4 °C in the equilibrating buffer. The enzyme content in this saturated gel was 25.5 μg of enzyme mg−1 of wet DEAE. The amount of enzyme in a defined weight of wet gel was obtained by eluting enzyme from the Chx saturated DEAE gel with 0.1 M acetate buffer, pH 4.5, and measuring the absorbency of the eluate at 280 nm.The DEAE–Chx immobilisation in PVA-SbQ was performed by mixing 25 μl of the Chx saturated gel with 25 μl of a PVA-SbQ solution in distilled water (1 + 4) and by spreading 20 μl of this solution on the glassy carbon electrode. The DEAE–Chx–PVA sensing layer was then dried, polymerised and stored under the same conditions as those described above.
Immobilised choline oxidase activity measurement
The activity of the different immobilised enzyme layers, that is the preactivated membranes, the PVA polymer and the DEAE–PVA polymer, were measured using a hydrogen peroxide amperometric sensor from INCELTECH-SGI (Toulouse, France). The H2O2 probe was composed of a platinum working electrode polarised at +650 mV vs. a platinum pseudo-reference electrode. The probe was connected to a PRGE type polarograph (Tacussel). The activity measurements were performed in 30 mM veronal buffer, pH 9, containing 30 mM KCl, 1.5 mM MgCl2 and choline chloride at a final concentration of 10 mM. The immobilised enzymatic activities were expressed in nmol min−1.Results and discussion
Effect of buffer and pH
As shown in previous studies, choline oxidase activity and H2O2 ECL detection are highly dependent on the buffer composition and on the pH value.4,15 The response of the ECL choline biosensor, assembled with Chx immobilised on ABC membranes, was then studied following the injection of 3 nmol of choline in a flowing stream composed of different buffers and at different pH values. Two different buffers: 0.1 M glycine buffer containing of 0.1 M KCl, and 30 mM veronal buffer containing 30 mM KCl and 1.5 mM MgCl2, have been tested in a pH range from 8 to 10.Fig. 2 presents the signal/noise (S/N) ratio variations with the pH for the buffers tested. The S/N ratio increases with pH and reaches a maximum value at pH 9 independently of the buffer used. Nevertheless, veronal buffer enables the achievement of a three times higher S/N ratio at this optimum pH value, and was then chosen as the carrier buffer solution for all subsequent studies.
 | ||
| Fig. 2 Choline electrochemiluminescent measurement in flow injection analysis system: S/N ratio as a function of the pH of the carrier solution. ECL measurements performed following the injection of 3 nmol of choline in (○) glycine buffer or (■) veronal buffer. Applied potential: +425 mV vs. a platinum pseudo-reference electrode. | ||
Effect of the flow rate and the stirring speed
It is well known that the flow rate and the stirring speed strongly influence the response of the FIA biosensor system involving immobilised enzyme membranes17 by modifying the residence time and the diffusional limitations, respectively.Thus, the effect of flow rate, from 0.08 to 0.82 ml min−1, on the cycle time and on the response of the choline ECL biosensor measured following the injection of 3 nmol of choline has been investigated. As shown in Fig. 3, increasing the flow rate from 0.08 to 0.82 ml min−1 not only induces a cycle time decrease from 22 min to 6 min but also a 80% decrease of the biosensor response, due to a decrease of the residence time of the sample in the flow cell. Consequently, the selection of an optimum flow rate appeared to be a compromise between the time needed to perform the measurement and the desired sensitivity of the analytical system. A 0.5 ml min−1 flow rate was then chosen as the optimum value, corresponding to an acceptable cycle time of 8 min.
 | ||
| Fig. 3 Effect of the flow rate on the cycle time (●) and on the S/N ratio (○) of the ECL choline biosensor. Applied potential: +425 mV vs. platinum pseudo-reference electrode. 3 nmol of choline are injected in the flowing stream (veronal buffer, pH 9). Stirring speed: 1200 rpm. | ||
Stirring efficiency is known to influence the diffusional limitation of the substrate and reaction products in heterogeneous enzymatic systems.17 Hence, in order to improve the detection limit obtained using the selected flow rate (0.5 ml min−1), the stirring efficiency in the 250 μl inner volume flow cell was studied (Fig. 4). The biosensor S/N ratio measured upon the injection of 3 nmol of choline increased with the stirring speed from 400 rpm to the optimum 1200 rpm and decreased for higher values which then generated an irregular stirring. A 1200 rpm optimum stirring speed was consequently chosen for all subsequent experiments.
 | ||
| Fig. 4 Effect of the stirring speed on the S/N ratio of the ECL choline biosensor. Applied potential: +425 mV vs. platinum pseudo-reference electrode. 3 nmol of choline are injected in the flowing stream (veronal buffer, pH 9). Flow rate: 0.5 ml min−1. | ||
Effect of the immobilisation support: use of different preactivated membranes
As shown in previous studies,10,15 the ability to retain active enzyme was highly dependent on the membrane structure and on its chemical reactivity. Similarly, both types of preactivated membranes, immobilised with the same Chx concentration, exhibit different total apparent activities, 1.26 nmol min−1 and 5.45 nmol min−1 for ABC and UltraBind type membrane disks of 7 mm, respectively.As shown in Table 1 the performances of the biosensor assembled with an UltraBind membrane are higher than the performances obtained with an ABC membrane, with detection limits of 75 pmol for the former and 300 pmol, for the latter.
The four times higher immobilised enzyme activity and the higher luminescence response of UltraBind membrane could be explained by the better yield of the immobilisation process.
Effect of the immobilisation support: use of a non-covalent immobilisation of Chx
As shown in the study mentioned above, the immobilised enzyme activity and the choline ECL biosensor performances could be modulated by changing the immobilisation support. Another approach has been also considered: the non-covalent immobilisation of choline oxidase by entrapment in a PVA-SbQ photopolymer directly coated onto the glassy carbon electrode. Such an immobilisation process has been already used for designing amperometric biosensors by direct polymerisation of the sensing layer on the transducer.7 In that case, the production of a high enzymatic activity-containing polymer was enabled and moreover, the response time of these electrochemical biosensors (few seconds) was largely reduced when compared to membrane-based biosensors.The ECL choline biosensor has been thus first modified with a Chx–PVA polymer directly deposited onto the glassy carbon electrode. As expected, the new ECL biosensor exhibited a really short cycle time of 3 min. Moreover, the apparent immobilised Chx activity measured, 9.25 nmol min−1, was higher than that obtained with the UltraBind membrane.
However, multiple injections of choline in the flow system demonstrated that the ECL signal measured decreased during the first 20 injections to reach then a constant value. At that time, the biosensor response remained stable and the performances of the biosensor presented in Table 1 were a detection limit of 220 pmol, and a linear range of detection over at least two decades of concentration.
This decrease of the ECL biosensor response observed for the first 20 injections was explained by the enzyme release from the PVA polymer due to its very wide meshes. In a flow system, in which the reaction medium is constantly renewed, this release appears to be a critical limitation to the use of PVA-SbQ polymer alone as the immobilisation support.
In order to overcome this problem of enzyme release, a two-step immobilisation procedure was developed making use of DEAE beads on which Chx was first tightly bound by ionic strength prior to the physical entrapment of the beads in the photopolymer.
The DEAE–Chx–PVA polymer then obtained and deposited onto the glassy carbon electrode (3 mm in diameter) exhibited a total apparent activity of 22 nmol min−1. The performances of the ECL biosensor associated with this novel bioactive layer are presented in Table 1. The detection limit was 10 pmol and a linear response was obtained over at least three decades of concentration. These performances were obtained directly after the introduction of the modified electrode in the flow cell, without washing or equilibration step. The use of DEAE beads to pre-bind the choline oxidase seemed thus to be a promising approach for obtaining high immobilised activity while avoiding the enzyme release from the photopolymer.
Operational stability of the different systems
The major biosensor requirements are sensitivity and stability. Sensitivity of the ECL biosensor been achieved in the above studies by selecting the most efficient Chx immobilisation procedure.Biosensors using both types of Chx immobilised preactivated membranes, ABC or UltraBind, exhibit lower operational stability. Indeed, when performing 60 successive injections of 3 nmol of choline, the measured ECL intensity was stable only for the first 45 assays with a mean value of 64 a.u. ± 6.3 a.u. and an RSD equal to 9.7%, and decreased progressively during the last 15 measurements.
Conversely, the electrochemiluminescence-based biosensor including the DEAE–Chx–PVA system exhibited a very good operational stability when performing 160 successive measurements, over a 3 d period (Fig. 5). A mean ECL signal of 267.5 a.u. ± 12 a.u., giving an RSD of 4.5%, was obtained for those 160 measurements. Moreover, no ECL signal variation was observed after two nights of dried storage of the sensing layer at 4 °C.
 | ||
| Fig. 5 Choline electrochemiluminescent measurement in flow injection analysis system: operational stability of the ECL biosensor. Applied potential: +425 mV vs platinum pseudo-reference electrode. 3 nmol of choline are injected in a pH 9 veronal buffer flowing stream. Flow rate: 0.5 ml min−1; stirring speed: 1200 rpm. The signal intensities from the 80th to the 95th injection show how the presence of an air bubble in the flowing stream can modify the ECL response. | ||
In conclusion, to develop a reliable ECL-based choline biosensor, commonly-used immobilisation methods (covalent binding on preactivated membranes and entrapment in a photopolymer) were evaluated as well as a new method consisting of pre-binding choline oxidase to DEAE Sepharose beads subsequently confined in a photopolymerised PVA film.
The biosensors based on preactivated, ABC type or UltraBind type membranes, exhibited the same operational stability of about 45 assays but different detection limits equal to 300 pmol, and 75 pmol, respectively.
The ECL biosensor based on direct entrapment of the choline oxidase in PVA was not adapted to flow injection conditions since the enzyme release from the sensing layer in contact with the flowing stream was shown to be high. Finally, the new immobilisation procedure developed, involving the concomitant use of anion exchanger beads (DEAE Sepharose) and a PVA photopolymer, appears to be promising as demonstrated by the very low detection limit obtained, and by the very good operational stability observed.
This detection limit of 10 pmol, corresponding to a 0.3 μM choline concentration in the sample injected in the system, is an acceptable value when compared with previous works1,4–6,8 based on electrochemical systems in which the best detection limit obtained was 1 μM. Moreover, previous studies performed with an ECL biosensor demonstrated that complex matrices such as human sera could be injected at a (1 + 9) dilution in the ECL biosensor without any interference problems.15 The present ECL biosensor appears to be an efficient way to perform sensitive detection of choline in a flow injection mode. Its extension to acetylcholine detection in complex media is now under investigation.
Acknowledgements
V. C. Tsafack acknowledges the financial support from the University of Bologna and from the EU within the framework of the Leonardo da Vinci programme.References
- C. Cremisini, S. Di Sario, J. Mela, R. Pilloton and G. Palleschi, Anal. Chim. Acta, 1995, 311, 273 CrossRef CAS.
- T. Ghous and A. Townshend, Anal. Chim. Acta, 1996, 332, 179 CrossRef CAS.
- A. Günther and U. Billitewski, Anal. Chim. Acta, 1995, 300, 117 CrossRef.
- R. M. Morelis and P. R. Coulet, Anal. Chim. Acta, 1990, 231, 27 CrossRef CAS.
- R. Rouillon, N. Mionetto and J. L. Marty, Anal. Chim. Acta, 1992, 268, 347 CrossRef CAS.
- J. L. Marty, K. Sode and I. Karube, Anal. Chim. Acta, 1990, 228, 49 CrossRef CAS.
- B. Leca, R. M. Morélis and P. R. Coulet, Mikrochim. Acta, 1995, 121, 147 CAS.
- H. Lapp, U. Spohn and D. Janasek, Anal. Lett., 1996, 29(1), 1.
- S. Luterotti and D. Maysinger, J. Pharm. Biomed. Anal., 1994, 12, 1083 CrossRef CAS.
- L. J. Blum, Bio- and Chemi-Luminescent Sensors, World Scientific, Singapore, 1997, pp. 96–155. Search PubMed.
- G. Bardeletti, F. Séchaud and P. R. Coulet, in Biosensor Principles and Applications, ed. L. J. Blum and P. R. Coulet, Marcel Dekker, New York, 1991, pp. 7–45. Search PubMed.
- L. J. Blum, Enzyme Microb. Technol., 1993, 15, 407 CrossRef CAS.
- A. Berger and L. J. Blum, Enzyme Microb. Technol., 1994, 16, 979 CrossRef CAS.
- S. Sakura, Anal. Chim. Acta, 1992, 262, 49 CrossRef CAS.
- C. A. Marquette and L. J. Blum, Anal. Chim. Acta, 1999, 381, 1 CrossRef CAS.
- C. A. Marquette, P. R. Coulet and L. J. Blum, Anal. Chim. Acta, 1999, 398, 173 CrossRef CAS.
- J. M. Engasser and C. Horvath, in Applied Biochemistry and Bioengineering, ed. L. B. Wingard, E. Katchalski-Katzir and L. Goldstein, Academic Press, New York, 1976, pp. 127–220. Search PubMed.
Footnote |
| † Present address: University of Bologna, Institute of Chemical Sciences, Via San Donato 15, 40127 Bologna, Italy. |
| This journal is © The Royal Society of Chemistry 2000 |
