Electron transport in crystalline PCBM-like fullerene derivatives: a comparative computational study†
Abstract
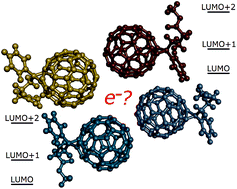
We present an extensive study of electron transport (ET) in several crystal forms of phenyl-C61-butyric acid methyl ester (PCBM) and 1-thienyl-C61-butyric acid methyl ester (ThCBM) fullerene derivatives. Our calculations are based on a localized representation of the electronic states. Orbital couplings, site energies and reorganization energies have been calculated using various density functional and semi-empirical techniques and used within the Landau–Zener, Marcus and Marcus–Levich–Jortner expressions to evaluate electron transfer rates. Electron mobilities have been then estimated by kinetic Monte Carlo (KMC) simulations. The adiabaticity of electron transfer directions within the different crystal structures has also been verified using the Landau–Zener expression. Finally, the role of low energy virtual orbitals of the fullerene molecules has been investigated using charge transport networks of increasing complexities. Our results show that these molecules may form one-, two- or three-dimensional percolation networks and that their higher energy orbitals often participate in ET. The highest mobility values were obtained for the crystal structure of ThCBM and are comparable to experimental values.

 Please wait while we load your content...
Please wait while we load your content...