Fe5C2 nanoparticles: a reusable bactericidal material with photothermal effects under near-infrared irradiation†
Abstract
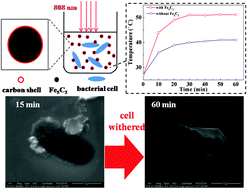
Hägg iron carbide (Fe5C2) was synthesized through a facile one-pot wet-chemical route and employed as a photothermal agent to inactivate bacterial cells. The as-prepared Fe5C2 nanoparticles (NPs) were about 20 nm in diameter, and exhibited strong magnetic properties (Ms = 122 emu g−1 at 298 K). Fe5C2 NPs exhibited excellent antibacterial capability toward both Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus) under near-infrared (NIR) irradiation. Under NIR irradiation, complete inactivation of E. coli and S. aureus cells (about 2 × 106 CFU per mL) could be obtained by 50 mg L−1 Fe5C2 NPs in 60 min and 150 min, respectively. Humic acid (HA) slightly inhibited the disinfection efficiency of Fe5C2 NPs, however, more than 99.9% of E. coli cells were inactivated in 60 min even when the concentration of HA was as high as 10 mg L−1. Complete disinfection of E. coli cells could be achieved with the presence of 10 mg L−1 HA by increasing the reaction time to 90 min. Moreover, Fe5C2 NPs showed great reusability, and complete disinfection of E. coli cells remained even after five consecutive reuse cycles. The increase in temperature of bacterial suspension caused by the photothermal effect of Fe5C2 NPs was determined to be the main reason for the inactivation of bacteria. Our study showed that Fe5C2 NPs have great application potential for bacterial disinfection in water.

 Please wait while we load your content...
Please wait while we load your content...