Identifying the anionic redox activity in cation-disordered Li1.25Nb0.25Fe0.50O2/C oxide cathodes for Li-ion batteries†
Abstract
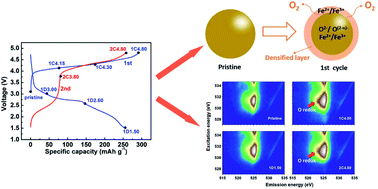
Li-excess cation-disordered cathodes for Li-ion batteries have attracted much interest due to their high capacity. However, issues regarding anionic redox activity and capacity degradation still need to be clarified for their performance improvement. In this work, a newly designed cation-disordered Li1.25Nb0.25Fe0.50O2/C cathode is investigated. The mapping of resonant inelastic X-ray scattering (mRIXS) reveals direct evidence of reversible anionic redox during cycling for the first time and defines the voltage range. Furthermore, differential electrochemical mass spectroscopy (DEMS) finds that oxygen release is involved during high voltage charging with electrolyte decomposition. A surface densified layer induced by excessive oxygen oxidation is also identified by in/ex situ X-ray diffraction (XRD). In the densified layer, lithium ion diffusion is blocked due to the reduced amount of percolating 0-TM (transition metal) network. Besides, irreversible oxygen loss during cycling leads to the sustained growth of the densified layer and results in larger lattice volume variations. Therefore, the performance degradation of the material is largely due to oxygen loss, surface densification, and large volume variation upon electrochemical operation.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...