Study of the surface reaction kinetics of (La,Sr)MnO3−δ oxygen carriers for solar thermochemical fuel production†
Abstract
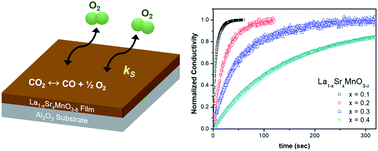
(La,Sr)MnO3−δ has received a great deal of attention as an oxygen carrier that can replace the state-of-the-art carrier CeO2 for solar-driven thermochemical fuel production. Despite the many relevant studies, however, the redox reaction kinetics of this material, which determines the fuel production rate, has rarely been reported. Here, we investigate the surface reaction rate of reduced Sr-doped lanthanum manganite thin films, as a model for a gas/solid interface of a perovskite-structured oxygen carrier under a condition, in which carbon monoxide is produced from CO2 in a two-step thermochemical cycling process. Thin films of La1−xSrxMnO3−δ (x = 0.1, 0.2, 0.3, 0.4) with dense and flat surfaces are fabricated via pulsed laser deposition, and their surface oxygen exchange rates are then characterized via electrical conductivity relaxation under actual operating conditions (T = 650 to 800 °C and pO2 = 2.9 × 10−19 to 9.0 × 10−13 atm). As the Sr content increases, the oxygen exchange greatly decelerates. On the other hand, for a given Sr content, the oxygen exchange does not vary much over a wide range of pO2 near the target temperature of 800 °C. We also observe the surface oxygen exchange rate has a direct impact on the CO production rate. These observations can guide the selection of an ideal oxygen carrier composition for high-performance fuel production.


 Please wait while we load your content...
Please wait while we load your content...