Cubic and octahedral Cu2O nanostructures as anodes for lithium-ion batteries
Abstract
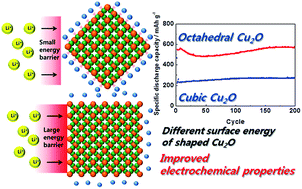
Well-defined nanostructured electrodes are known to have improved lithium ion reaction properties for lithium-ion batteries. Herein, we prepared shape-controlled Cu2O nanostructures as an anode material using ascorbic acid as a reducing agent with and without polyvinylpyrrolidone (PVP) as a surfactant. Using scanning electron microscopy, transmission electron microscopy, and X-ray diffraction methods, we observed that the sample prepared in the absence of PVP exhibited cubes with dominant {100} facets, whereas octahedral Cu2O nanostructures with dominant {111} facets were formed in the presence of PVP. During the charge–discharge process, an octahedron-shaped Cu2O nanostructured electrode having {111} facets favourable for lithium ion transport revealed an enhanced conversion reaction mechanism with high reversible capacity and high rate cycling performance, due to its low charge transfer resistance and high lithium ion diffusion coefficient.

 Please wait while we load your content...
Please wait while we load your content...