Photocatalytic splitting of water on s-triazine based graphitic carbon nitride: an ab initio investigation†
Abstract
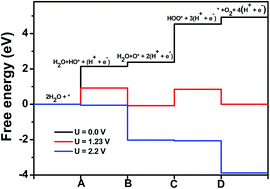
Density functional theory based investigations have been carried out to understand the reaction mechanism of the overall photocatalytic water splitting reaction on s-triazine based carbon nitride (g-CN) and to calculate the overpotentials for both oxygen and hydrogen evolution reactions. The calculated free energy changes for different possible intermediate reactions show that at the equilibrium potential of 1.23 V (against the NHE), the oxygen evolution reaction is not completely downhill indicating that the photo-generated holes at 1.23 V cannot oxidize water to oxygen. The oxygen evolution reaction on the g-CN surface is however found to be completely downhill at and above the potential of 2.16 V. As the valence band of g-CN is shown to be located at a potential of 2.64 V, the photo-generated holes in the valence band can oxidise water to oxygen without the aid of any co-catalyst. However, the hydrogen evolution reaction is found to have an overpotential of around 1.1 V and the photo-generated electrons in the conduction band of g-CN are placed at around 0.26 V above the hydrogen reduction potential indicating that the hydrogen evolution is possible only in the presence of a co-catalyst.

 Please wait while we load your content...
Please wait while we load your content...