A novel electroactive hybrid film electrode with proton buffer effect for detecting hydrogen peroxide and uric acid
Abstract
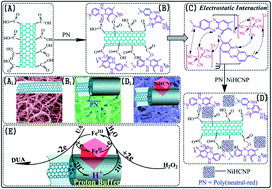
In this study, a novel hybrid film electrode with proton buffer effect for the detection of hydrogen peroxide (H2O2) and uric acid (UA) was fabricated by a facile one-step electrosynthesis method using cyclic voltammetry (CV). The electrode was composed of a nickel hexacyanoferrate cubic nanoparticle (NiHCNP) inlaid poly(neutral-red) (PN) film formed on carbon nanotube-(CNTs) modified glassy carbon (GC) plate. PN in the hybrid film was found to not only act as a proton buffer, which can receive and release protons to meet the demands of electrocatalytic reaction, but also to anchor NiHCNP on the surface of CNTs and stabilize NiHCNP. The morphology and structure of the as-prepared NiHCNP/PN/CNTs hybrid film was characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectrum (EDS), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR). The prepared hybrid film electrodes showed excellent electrocatalytic activity with high sensitivity, fast response time and low detection limit for the detection of H2O2 as well as UA.

 Please wait while we load your content...
Please wait while we load your content...