Selective lithium extraction from brines by chemical reaction with battery materials†
Abstract
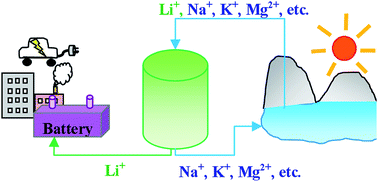
We demonstrate fast and efficient chemical redox insertion of lithium ions into solid FePO4 from lithium salt solutions contaminated with other cations. The method is illustrated with sodium thiosulfate, Na2S2O3, as a reducing agent that is found to have an optimum redox potential for this reaction. The method shows a very high selectivity for lithium extraction; enrichment in lithium concentration vs. other ions of more than 500 is achieved under the conditions relevant to lithium extraction from brines.

 Please wait while we load your content...
Please wait while we load your content...