Effect of preparation conditions on the structural and acid catalytic properties of protonated titanate nanotubes†
Abstract
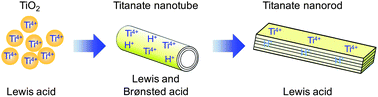
The effect of hydrothermal treatment on the morphological and acid catalytic properties of titanate nanomaterials was examined. Anatase nanoparticles were converted into titanate nanotubes and layered titanate nanorods according to the heating temperature used during the hydrothermal treatment. Fourier transform infrared (FT-IR) spectroscopy measurements and acid-base titrations revealed that all titanate nanomaterials possess Lewis acid sites, whereas Brønsted acid sites are formed after the hydrothermal treatment at temperatures higher than 323 K. At 423 K, titanate nanotubes with an inner diameter of 5 nm were formed, which exhibited the highest catalytic activity for the liquid phase Friedel–Crafts alkylation of aromatics with benzyl chloride or benzyl alcohol. The titanate nanorods prepared at 473 K exhibited Lewis acidity, but had no effective Brønsted acid sites regardless of the high ion-exchange capacity. The high catalytic performance of the titanate nanotubes can be attributed to the presence of a large number of both Lewis and Brønsted acid sites located on the surfaces of the nanotubes.

 Please wait while we load your content...
Please wait while we load your content...