New directions for hydrogen storage: sulphur destabilised sodium aluminium hydride†
Abstract
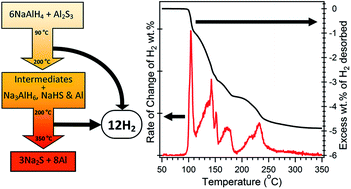
Aluminium sulphide (Al2S3) is predicted to effectively destabilise sodium aluminium hydride (NaAlH4) in a single-step endothermic hydrogen release reaction. The experimental results show unexpectedly complex desorption processes and a range of new sulphur containing hydrogen storage materials have been observed. The NaAlH4–Al2S3 system releases a total of 4.9 wt% of H2 that begins below 100 °C without the need for a catalyst. Characterisation via temperature programmed desorption, in situ synchrotron powder X-ray diffraction, ex situ x-ray diffraction, ex situ Fourier transform infrared spectroscopy and hydrogen sorption measurements reveal complex decomposition processes that involve multiple new sulphur-containing hydride compounds. The system shows partial H2 reversibility, without the need for a catalyst, with a stable H2 capacity of ∼1.6 wt% over 15 cycles in the temperature range of 200 °C to 300 °C. This absorption capacity is limited by the need for high H2 pressures (>280 bar) to drive the absorption process at the high temperatures required for reasonable absorption kinetics. The large number of new phases discovered in this system suggests that destabilisation of complex hydrides with metal sulphides is a novel but unexplored research avenue for hydrogen storage materials.

 Please wait while we load your content...
Please wait while we load your content...