Electrocatalytic hydrogen evolution with gallium hydride and ligand-centered reduction†
Abstract
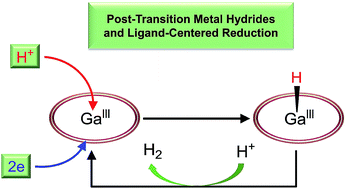
GaIII chloride tetrakis(pentafluorophenyl)porphyrin (1) was synthesized and shown to be a highly active and stable post-transition metal-based electrocatalyst for the hydrogen evolution reaction (HER). Electrochemical and spectroscopic studies indicate that both the first and second reduction events of 1 are ligand-centered. The 2e-reduced form 12− reacts with a proton to give GaIII–H species (1–H), which undergoes protonolysis with Brønsted acids to produce H2. The identification of key intermediates 1−, 12−, and 1–H leads to a catalytic cycle, which is valuable for the fundamental understanding of the HER. This study presents a rare but highly active HER electrocatalyst with unusual features, including ligand-centered electron transfer and formation of post-transition metal hydride.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...