Ni-catalysed reductive arylalkylation of unactivated alkenes†
Abstract
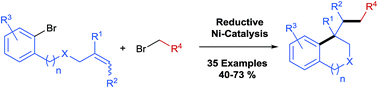
In this protocol Ni-catalysed reductive arylalkylation of unactivated alkenes tethered to aryl bromides with primary alkyl bromides has been accomplished, providing a new path to construct diverse benzene-fused carbo- and heterocyclic cores including indanes, tetrahydroisoquinolines, indolines and isochromanes. Notably, this new method circumvents the pregeneration of organometallics and demonstrates high tolerance to a wide range of functional groups. The preliminary mechanistic investigations suggest a reaction pathway with an intermediate reduction.
- This article is part of the themed collection: Most popular 2018-2019 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...