Oxygen transfer in electrophilic epoxidation probed by 17O NMR: differentiating between oxidants and role of spectator metal oxo†
Abstract
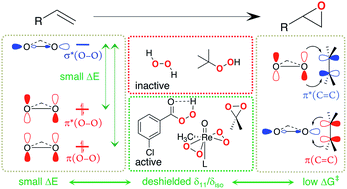
Peroxide compounds are used both in laboratory and industrial processes for the electrophilic epoxidation of olefins. Using NMR-spectroscopy, we investigate why certain peroxides engage in this type of reaction while others require activation by metal catalysts, e.g. methyltrioxorhenium (MTO). More precisely, an analysis of 17O NMR chemical shift and quadrupolar coupling parameters provides insights into the relative energy of specific frontier molecular orbitals relevant for reactivity. For organic peroxides or H2O2 a large deshielding is indicative of an energetically high-lying lone-pair on oxygen in combination with a low-lying σ*(O–O) orbital. This feature is particularly pronounced in species that engage in electrophilic epoxidation, such as peracids or dimethyldioxirane (DMDO), and much less pronounced in unreactive peroxides such as H2O2 and ROOH, which can however be activated by transition-metal catalysts. In fact, for the proposed active peroxo species in MTO-catalyzed electrophilic epoxidation with H2O2 an analysis of the 17O NMR chemical shift highlights specific π- and δ-type orbital interactions between the so-called metal spectator oxo and the peroxo moieties that raise the energy of the high-lying lone-pair on oxygen, thus increasing the reactivity of the peroxo species.


 Please wait while we load your content...
Please wait while we load your content...