A robust iron catalyst for the selective hydrogenation of substituted (iso)quinolones†
Abstract
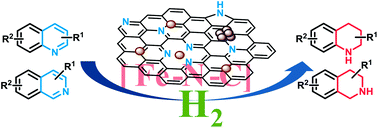
By applying N-doped carbon modified iron-based catalysts, the controlled hydrogenation of N-heteroarenes, especially (iso)quinolones, is achieved. Crucial for activity is the catalyst preparation by pyrolysis of a carbon-impregnated composite, obtained from iron(II) acetate and N-aryliminopyridines. As demonstrated by TEM, XRD, XPS and Raman spectroscopy, the synthesized material is composed of Fe(0), Fe3C and FeNx in a N-doped carbon matrix. The decent catalytic activity of this robust and easily recyclable Fe-material allowed for the selective hydrogenation of various (iso)quinoline derivatives, even in the presence of reducible functional groups, such as nitriles, halogens, esters and amides. For a proof-of-concept, this nanostructured catalyst was implemented in the multistep synthesis of natural products and pharmaceutical lead compounds as well as modification of photoluminescent materials. As such this methodology constitutes the first heterogeneous iron-catalyzed hydrogenation of substituted (iso)quinolones with synthetic importance.


 Please wait while we load your content...
Please wait while we load your content...