Gold-catalyzed conversion of lignin to low molecular weight aromatics†
Abstract
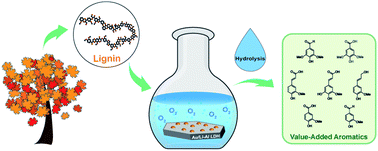
A heterogeneous catalyst system, employing Au nanoparticles (NPs) and Li–Al (1 : 2) layered double hydroxide (LDH) as support, showed excellent activity in aerobic oxidation of the benzylic alcohol group in β-O-4 linked lignin model dimers to the corresponding carbonyl products using molecular oxygen under atmospheric pressure. The synergistic effect between Au NPs and the basic Li–Al LDH support induces further reaction of the oxidized model compounds, facilitating facile cleavage of the β-O-4 linkage. Extension to oxidation of γ-valerolactone (GVL) extracted lignin and kraft lignin using Au/Li–Al LDH under similar conditions produced a range of aromatic monomers in high yield. Hydrolysis of the Au/Li–Al LDH oxidized lignin was found to increase the degree of lignin depolymerization, with monomer yields reaching 40% for GVL extracted lignin. Based on these results, the Au/Li–Al LDH + O2 catalyst system shows potential to be an environmentally friendly means of depolymerizing lignin to low molecular weight aromatics under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...