Divergent enantioselective synthesis of hapalindole-type alkaloids using catalytic asymmetric hydrogenation of a ketone to construct the chiral core structure†
Abstract
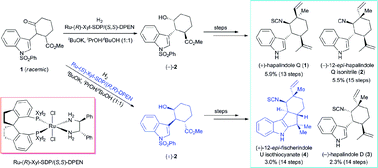
A divergent enantioselective approach to hapalindole-type alkaloids is described. The route features a ruthenium-catalyzed asymmetric hydrogenation of a ketone via DKR to construct the chiral trans-1-indolyl-2-isopropenylcyclohexane skeleton and a switchable sequence of methylation and acetylation/aldol reaction to access a chiral quaternary stereocenter. (+)-Hapalindole Q (1, 13 steps, 5.9% overall yield), (−)-12-epi-hapalindole Q isonitrile (2, 15 steps, 5.5% overall yield), (−)-hapalindole D (3, 14 steps, 2.3% overall yield), and (+)-12-epi-fischerindole U isothiocyanate (4, 14 steps, 3.0% overall yield) were synthesized in 13–15 steps from a commercially available material to demonstrate the application of this approach.

 Please wait while we load your content...
Please wait while we load your content...