The third orthogonal dynamic covalent bond†
Abstract
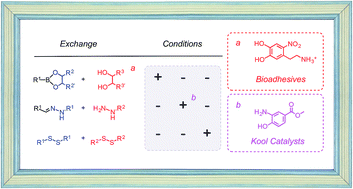
Orthogonal dynamic covalent bonds are of interest for the construction of functional systems. The orthogonality of disulfide and hydrazone exchange under basic and acidic conditions, respectively, is well established. However, the integration of boronate esters as the third bond has failed so far because they exchanged too easily, especially under hydrazone exchange conditions. In this report, a collection of bioinspired catechols derived from adhesive natural products from cyanobacteria is screened with phenylboronic acids with proximal alcohols (benzoboroxoles), amines and fluorines to identify the least labile boronate esters. Moreover, Kool's 2-aminophenol catalysts are introduced to selectively accelerate hydrazone exchange without disturbing sufficiently inert boronate esters. Based on these results, we identified three different conditions to selectively exchange disulfides, hydrazones and boronate esters, that is to demonstrate the existence of three orthogonal dynamic covalent bonds. Moreover, their compatibility with functional systems is confirmed by successful hydrazone exchange in multicomponent surface architectures in the presence of intact boronate esters and disulfides.
- This article is part of the themed collection: ISACS18: Challenges in Organic Materials and Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...