Structure and assembly mechanisms of toxic human islet amyloid polypeptide oligomers associated with copper†
Abstract
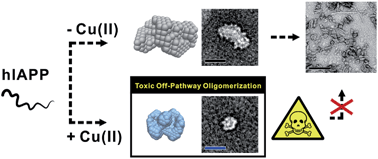
Amyloidosis is a clinical disorder implicated with the formation of toxic amyloid aggregates. Despite their pathological significance, it is challenging to define the structural characteristics of amyloid oligomers owing to their metastable nature. Herein, we report structural and mechanistic investigations of human islet amyloid polypeptide (hIAPP) oligomers, found in type II diabetes mellitus, in both the absence and presence of disease-relevant metal ions [i.e., Cu(II) and Zn(II)]. These metal ions show suppressive effects on hIAPP fibrillation and facilitate the generation of toxic oligomers. Using circular dichroism spectroscopy, transmission electron microscopy, gel electrophoresis, small-angle X-ray scattering, and ion mobility-mass spectrometry, we investigated the assembly mechanisms of hIAPP oligomers in the presence and absence of metal ions. Oligomerization of both metal-free hIAPP and metal-associated hIAPP monomers is initiated following a similar growth model. However, in the presence of Cu(II), hIAPP monomers self-assemble into small globular aggregates (Rg ∼ 45 Å) with a random coil structure. This Cu(II)-associated hIAPP oligomer shows an off-pathway aggregation, and is suggested to be an end product which is toxic to pancreatic β-cells. On the other hand, metal-free hIAPP and Zn(II)-associated hIAPP monomers generate relatively less toxic aggregates that eventually grow into fibrils. We suggest that the coordination of hIAPP to Cu(II) and the relatively high stability (Ka, ca. 108 M−1) of hIAPP–Cu(II) complexes result in the abnormal conformation and toxicity of hIAPP oligomers. Overall, through combining multiple biophysical methods, our studies suggest that molecular interactions between hIAPP and Cu(II) induce a different pathway for hIAPP assembly. This work will advance our knowledge of the conformational basis, assembly mechanism, and toxicity of small soluble amyloid oligomers.

 Please wait while we load your content...
Please wait while we load your content...