A case study of proton shuttling in palladium catalysis†
Abstract
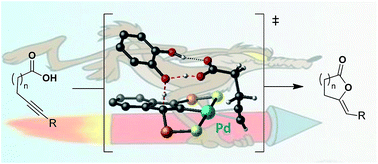
The mechanism of alkynoic acid cycloisomerization with SCS indenediide Pd pincer complexes has been investigated experimentally and computationally. These studies confirmed the cooperation between the Pd center and the backbone of the pincer ligand, and revealed the involvement of a second molecule of substrate. It acts as a proton shuttle in the activation of the acid, it directs the nucleophilic attack of the carboxylic acid on the π-coordinated alkyne and it relays the protonolysis of the resulting vinyl Pd species. A variety of H-bond donors have been evaluated as external additives, and polyols featuring proximal hydroxyl groups, in particular catechol derivatives, led to significant catalytic enhancement. The impact of 4-nitrocatechol and 1,2,3-benzenetriol is particularly striking on challenging substrates such as internal 4- and 5-alkynoic acids. Endo/exo selectivities up to 7.3/1 and 60-fold increase in reactivity were achieved.

 Please wait while we load your content...
Please wait while we load your content...