Effects of reagent rotation on interferences in the product angular distributions of chemical reactions†
Abstract
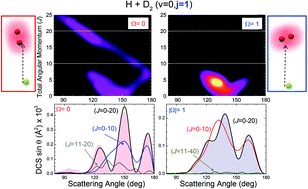
Differential cross sections (DSCs) of the HD(v′, j′) product for the reaction of H atoms with supersonically cooled D2 molecules in a small number of initial rotational states have been measured at a collision energy of 1.97 eV. These DCSs show an oscillatory pattern that results from interferences caused by different dynamical scattering mechanisms leading to products scattered into the same solid angle. The interferences depend on the initial rotational state j of the D2(v = 0, j) reagent and diminish in strength with increasing rotation. We present here a detailed explanation for this behavior and how each dynamical scattering mechanism has a dependence on the helicity Ω, the projection of the initial rotational angular momentum j of the D2 reagent on the approach direction. Each helicity corresponds to a different internuclear axis distribution, with the consequence that the dependence on Ω reveals the preference of the different quasiclassical mechanisms as a function of approach direction. We believe that these results are general and will appear in any reaction for which several mechanisms are operative.


 Please wait while we load your content...
Please wait while we load your content...