Controllability of dynamic double helices: quantitative analysis of the inversion of a screw-sense preference upon complexation†
Abstract
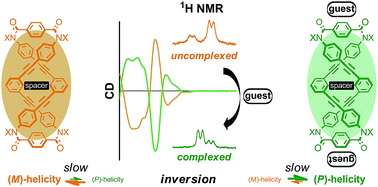
We describe a quantitative analysis of the complexation-induced inversion of a screw-sense preference based on a conformationally dynamic double-helix structure in a macrocycle. The macrocycle is composed of two twisting units (terephthalamide), which are spaced by two strands (1,3-bis(phenylethynyl)benzene), and is designed to generate a double-helix structure through twisting about a C2 axis in a conrotatory manner. The attachment of chiral auxiliaries to the twisting units induces a helical preference for a particular sense of (M)- or (P)-helicity through the intramolecular transmission of chirality to dynamic double helices. The twisting unit can also act as a binding site for capturing a guest molecule, and, in a complexed state, the preferred screw sense of the dynamic double-helix structure is reversed to exhibit the contrary preference. We quantitatively monitored the complexation-induced inversion of the screw-sense preference using 1H NMR spectroscopy, which enabled us to observe independently two species with (M)- or (P)-helicity in both the absence and presence of a guest molecule. Inversion of the screw-sense preference was induced upon complexation with an achiral guest as well as a chiral guest.

 Please wait while we load your content...
Please wait while we load your content...