Mixed-valent, heteroleptic homometallic diketonates as templates for the design of volatile heterometallic precursors†
Abstract
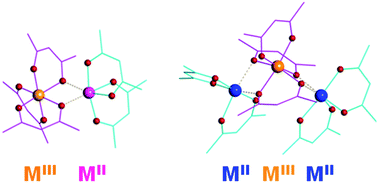
A novel series of mixed-valent, heteroleptic transition metal diketonates that can be utilized as prospective single-source precursors for the low-temperature preparation of oxide materials are reported. The first mixed-valent iron β-diketonates with different FeIII/FeII ratios have been synthesized by applying the mixed-ligand approach. Based on nearly quantitative reaction yields and analysis of iron–oxygen bonds, these compounds were formulated as [FeIII(acac)3][FeII(hfac)2] (1) and [FeII(hfac)2][FeIII(acac)3][FeII(hfac)2] (2). In the above heteroleptic complexes, the Lewis acidic, coordinatively unsaturated FeII centers chelated by two hfac (hexafluoroacetylacetonate) ligands with electron-withdrawing substituents maintain bridging interactions with oxygen atoms of electron-donating acac (acetylacetonate) groups that chelate the neighboring FeIII atoms. Switching the ligands on FeIII and FeII atoms in starting reagents resulted in the instant ligand exchange between iron centers and in yet another polynuclear homometallic diketonate [FeII(hfac)2][FeIII(acac)2(hfac)][FeII(hfac)2] (3) that adheres to the same bonding pattern as in complexes 1 and 2. The proposed synthetic methodology has been extended to design heterometallic diketonates with different M : M′ ratios. Homometallic parent molecules have been used as templates to obtain heterometallic mixed-valent [FeIII(acac)3][MnII(hfac)2] (4) and [NiII(hfac)2][FeIII(acac)3][NiII(hfac)2] (5) complexes. The combination of two different diketonate ligands with electron-donating and electron-withdrawing substituents was found to be crucial for maintaining the above mixed-valent heterometallic assemblies. Theoretical investigation of two possible “isomers”, [FeIII(acac)3][MnII(hfac)2] (4) and [MnIII(acac)3][FeII(hfac)2] (4′) provided an additional support for the metal site assignment giving a preference of 9.78 kcal mol−1 for the molecule 4. Heterometallic complexes obtained in the course of this study have been found to act as effective single-source precursors for the synthesis of mixed-transition metal oxide materials MxM′2−xO3 and MxM′1−xO. The title highly volatile precursors can be used for the low-temperature preparation of both amorphous and crystalline heterometallic oxides in the form of thin films or nanosized particles that are known to operate as efficient catalysts in oxygen evolution reaction.

 Please wait while we load your content...
Please wait while we load your content...