Electrode initiated proton-coupled electron transfer to promote degradation of a nickel(ii) coordination complex†
Abstract
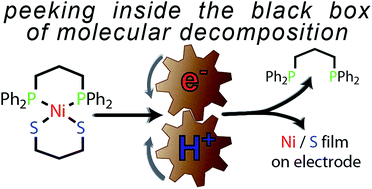
A Ni(II) bisphosphine dithiolate compound degrades into an electrode-adsorbed film that can evolve hydrogen under reducing and protic conditions. An electrochemical study suggests that the degradation mechanism involves an initial concerted proton–electron transfer. The potential susceptibility of Ni–S bonds in molecular hydrogen evolution catalysts to degradation via C–S bond cleavage is discussed.
- This article is part of the themed collection: Global Energy Challenges: Hydrogen Energy

 Please wait while we load your content...
Please wait while we load your content...