Rational design of small indolic squaraine dyes with large two-photon absorption cross section†
Abstract
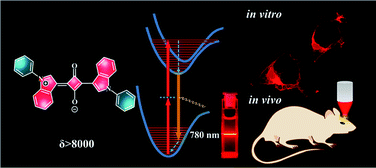
Small organic dyes with large two-photon absorption (TPA) cross sections (δ) are more desirable in many applications compared with large molecules. Herein, we proposed a facile theoretical method for the fast screening of small organic molecules as potential TPA dyes. This method is based on a theoretical analysis to the natural transition orbitals (NTOs) directly associated with the TPA transition. Experimental results on the small indolic squaraine dyes (ISD) confirmed that their TPA cross sections is strongly correlated to the delocalization degree of the NTOs of the S2 excited states. Aided by this simple and intuitive method, we have successfully designed and synthesized a small indolic squaraine dye (ISD) with a remarkable δ value above 8000 GM at 780 nm. The ISD dye also exhibits a high singlet oxygen generation quantum yield about 0.90. The rationally designed TPA dye was successfully applied in both two-photon excited fluorescence cell imaging and in vivo cerebrovascular blood fluid tracing.

 Please wait while we load your content...
Please wait while we load your content...