Twisting and piezochromism of phenylene-ethynylenes with aromatic interactions between side chains and main chains†
Abstract
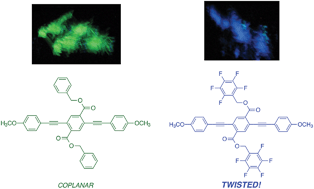
This paper describes a series of three-ring phenylene-ethynylenes (PEs) in which specific, non-covalent arene–arene interactions control conformation in the solid-state. As determined by single crystal X-ray structures, edge-face interactions between benzyl ester side chains and conjugated main chains are observed. In contrast, perfluorobenzyl ester side chains interact cofacially with main chains, resulting in ∼60° torsional angles between neighboring aryl rings in crystalline PEs. Absorbance and fluorescence spectra of films of these compounds reflect these conformational effects, with the spectra of perfluorobenzyl-substituted compounds shifting hypsochromically from solution- to solid-state. In a demonstration of how balancing non-covalent interactions can open the way to new responsive materials, a main chain twisted derivative with octyloxy substituents displayed significant piezochromic behavior.

 Please wait while we load your content...
Please wait while we load your content...