Solid phase extraction based on the phase transition of poly(N-isopropylacrylamide): the extraction behaviour of lanthanide(iii) ions in highly acidic solutions†
Abstract
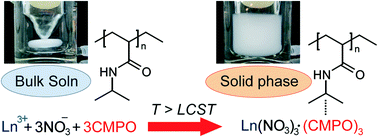
The present study investigates the applicability of poly(N-isopropylacrylamide) (PNIPAAm) as an extraction medium for trivalent lanthanide (Ln(III)) ions in high concentrations of nitric acid solutions by using octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO) as an extracting agent. The extraction is based on the hydrophobic, co-aggregative binding of the Ln-CMPO nitrate complex formed in situ onto the PNIPAAm aggregates growing above the phase-transition temperature. The resulting co-aggregates enabled easy separation of the liquid and solid phases. The extraction of Ln(III) ions was affected by the efficiency and extent of complexation and the stability of the complex. Furthermore, the difference in the stability of Ln-CMPO complex species allowed for the stripping of Ln(III) ions in hydrochloric acid solutions.


 Please wait while we load your content...
Please wait while we load your content...