External electric field: a new catalytic strategy for the anti-Markovnikov hydrohydrazination of parent hydrazine†
Abstract
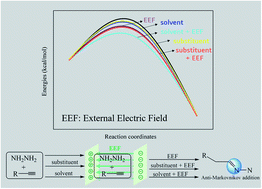
The anti-Markovnikov hydroamination reaction is considered to be a particular challenge, and one of the reactants, parent hydrazine, is also regarded as a troubling reagent. In this study, we first studied the hydrohydrazination of parent hydrazine via an effective and green catalyst—external electric field (EEF). The calculation results demonstrated that the anti-Markovnikov and Markovnikov pathways are competitive when there was no catalyst. EEF oriented along the negative direction of the X axis (Fx) accelerated the anti-Markovnikov addition reaction. Moreover, it lowered the barrier height of the first step by 16.0 kcal mol−1 (from 27.8 to 11.8 kcal mol−1) when the field strength was 180 (×10−4) au. Under the same conditions, the Markovnikov reaction pathway was inhibited, which means that EEF achieved the specificity of hydrohydrazination. The solvents are favorable for the first step addition reaction, particularly the synergy between solvents and Fx lowered the barrier heights by 8.3 (C6H6) and 10.7 (DMSO) kcal mol−1 for an Fx = −60 (×10−4) au. Besides, the introduction of the electron-withdrawing substituent (trifluoromethyl) is also a good strategy to catalyze hydrohydrazination, while the electron-donating group (methoxy) is unfavorable.


 Please wait while we load your content...
Please wait while we load your content...