An efficient and scalable synthesis of 2,4-di-N-acetyl-l-altrose (l-2,4-Alt-diNAc)†
Abstract
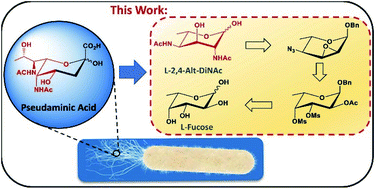
Bacterial nonulosonic acids such as pseudaminic acids and others constitute a family of 9-carbon monosaccharides that contain a common 3-deoxy-2-ketoacid fragment but differ in their stereochemistries at 5 stereogenic centers between C-4 to C-8. Their unique structures make them attractive targets for use as antigens in vaccinations to combat drug-resistant bacterial infections and their challenging stereochemistries have attracted considerable attention from chemists. In this work we report the development of an improved synthesis for 2,4-di-N-acetyl-L-altrose (L-2,4-Alt-diNAc), which is a key hexose required for the chemical and chemoenzymatic synthesis of pseudaminic acids. Using L-fucose as a starting material, our synthesis overcomes several pitfalls in previously reported syntheses.


 Please wait while we load your content...
Please wait while we load your content...