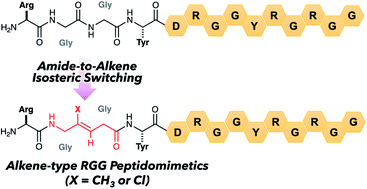
Stereoselective synthesis of Gly-Gly-type (E)-methylalkene and (Z)-chloroalkene dipeptide isosteres and their application to 14-mer RGG peptidomimetics†
Abstract
Stereoselective and efficient synthesis of Gly-Gly-type (E)-methylalkene and (Z)-chloroalkene dipeptide isosteres is realized by organocuprate-mediated single electron transfer reduction. The synthetic isosteres can be used in Fmoc-based solid phase peptide synthesis, resulting in the preparation of the 14-mer RGG peptidomimetics containing an (E)-methylalkene or a (Z)-chloroalkene unit.


 Please wait while we load your content...
Please wait while we load your content...