An environment-friendly crosslinked binder endowing LiFePO4 electrode with structural integrity and long cycle life performance†
Abstract
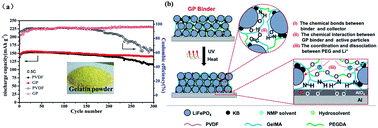
Lithium iron phosphate (LiFePO4) is one of the most widely used cathode materials of lithium ion batteries. However, its commercial binder polyvinylidene fluoride (PVDF) is costly, less environmental-friendly and unstable during the long cycling process because of the weak van der Waals forces between the PVDF binder and electrode materials. Herein, an aqueous binder was designed using methacrylate-modified gelatin through UV photo-crosslinking. The crosslinked network and specific functional groups (carboxyl and amino) of the gelatin binder are superior in stabilizing the LiFePO4 electrode structure during long cycles by mitigating the formation of cracks and suppressing the detachment of electrode materials from the Al current collector. The LiFePO4 electrode with gelatin binder displays a high capacity of 140.3 mA h g−1 with 90.1% retention after 300 cycles at 0.5C, which are both superior to that of the PVDF binder (only 114.4 mA h g−1 and 74.8%). This work provides a promising binder to replace the commercial PVDF binder for practical application in energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...