Catalyst-free visible-light-induced condensation to synthesize bis(indolyl)methanes and biological activity evaluation of them as potent human carboxylesterase 2 inhibitors†
Abstract
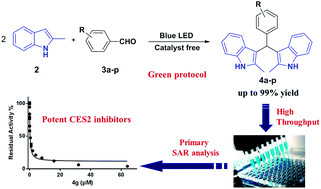
A mild strategy for visible-light-induced synthesis of bis(indolyl)methanes was developed using aromatic aldehydes and indole as substrates. This reaction could be performed at room temperature under catalyst- and additive-free conditions to synthesize a series of bis(indolyl)methanes in good to excellent yields. In addition, all synthesized bis(indolyl)methanes together with β-substituted indole derivatives synthesized according to our previous work, were evaluated for their inhibitory effect against human carboxylesterase (CES1 and CES2). Primary structure–activity relationship analysis of all tested compounds showed that the modifications of β-substituted indole at the β-site with another indolyl group led to a significant enhancement of the inhibitory effect on CES2, and the bisindolyl structure is essential for CES2 inhibition. These results demonstrated that these bis(indolyl)methanes are potent and selective CES2 inhibitors, which might be helpful for medicinal chemists to design and develop more potent and selective CES2 inhibitors for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...