Pd-catalyzed intramolecular addition of active methylene compounds to alkynes with subsequent cross-coupling with (hetero)aryl halides†
Abstract
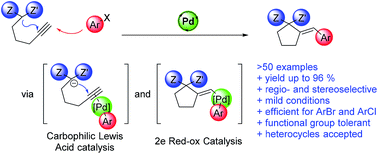
We report an efficient protocol for tandem Pd-catalyzed intramolecular addition of active methylene compounds to alkynes, followed by subsequent cross-coupling with (hetero)aryl bromides and chlorides. The reaction proceeds under mild conditions, providing excellent functional group tolerance, including unprotected OH, NH2 groups, enolizable ketones, or a variety of heterocycles. Mechanistic studies point towards a catalytic cycle involving oxidative addition, intramolecular nucleophilic addition to the Pd(II)-activated alkyne, and reductive elimination, with 5-exo-dig cyclization being the rate limiting step.


 Please wait while we load your content...
Please wait while we load your content...