A molecular electron density theory study of the mechanism, chemo- and stereoselectivity of the epoxidation reaction of R-carvone with peracetic acid†
Abstract
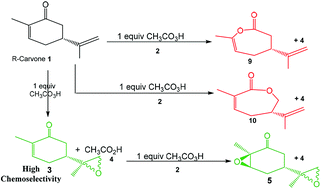
The epoxidation reaction of R-carvone 8 with peracetic acid 9 has been studied within the molecular electron density theory at the B3LYP/6-311(d,p) computational level. The chemo- and stereoisomeric reaction paths involving the two C–C double bonds of R-carvone 8 have been studied. DFT calculations account for the high chemoselectivity involving the C–C double bond of the isopropenyl group and the low diastereoselectivity, in complete agreement with the experimental outcomes. The Baeyer–Villiger reaction involving the carbonyl group of R-carvone 8 has also been analysed. A bonding evolution theory analysis of the epoxidation reaction shows the complexity of the bonding changes taking place along this reaction. Formation of the oxirane ring takes place asynchronously at the end of the reaction by attack of anionic oxygen on the two carbons of the isopropenyl C–C double bond.


 Please wait while we load your content...
Please wait while we load your content...